The Molecular Basis of Organic Chemiluminescence
- PMID: 37185527
- PMCID: PMC10136088
- DOI: 10.3390/bios13040452
The Molecular Basis of Organic Chemiluminescence
Abstract
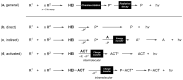
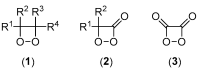
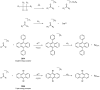
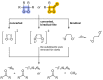
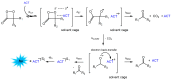
Bioluminescence (BL) and chemiluminescence (CL) are interesting and intriguing phenomena that involve the emission of visible light as a consequence of chemical reactions. The mechanistic basis of BL and CL has been investigated in detail since the 1960s, when the synthesis of several models of cyclic peroxides enabled mechanistic studies on the CL transformations, which led to the formulation of general chemiexcitation mechanisms operating in BL and CL. This review describes these general chemiexcitation mechanisms-the unimolecular decomposition of cyclic peroxides and peroxide decomposition catalyzed by electron/charge transfer from an external (intermolecular) or an internal (intramolecular) electron donor-and discusses recent insights from experimental and theoretical investigation. Additionally, some recent representative examples of chemiluminescence assays are given.
Keywords: 1,2-dioxetanes; 1,2-dioxetanones; bioluminescence; chemiluminescence; cyclic peroxides; sensors; ultrasensitive detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

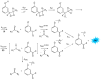
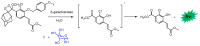



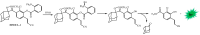

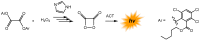
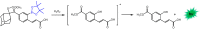
References
-
- El Seoud O.A., Baader W.J., Bastos E.L. In: Encyclopedia of Physical Organic Chemistry, 5 Volume Set. Wang Z., editor. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016.
-
- Baader W.J., Stevani C.V., Bastos E.L. Chemiluminescence of Organic Peroxides. In: Rappoport Z., editor. The Chemistry of Peroxides. Wiley-VCH; Chichester, UK: 2006. pp. 1211–1278.
Publication types
MeSH terms
Substances
Grants and funding
- 2014/22136-4/São Paulo Research Foundation
- 2022/02183-4/São Paulo Research Foundation
- 303015/2019-5/National Council for Scientific and Technological Development
- 303015/2019-5/National Council for Scientific and Technological Development
- Graduate Program of Chemistry, IQUSP (Finance Code 001)/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources

