Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications
- PMID: 37185528
- PMCID: PMC10136782
- DOI: 10.3390/bios13040453
Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications
Abstract
Electrochemical sensors consisting of screen-printed electrodes (SPEs) are recurrent devices in the recent literature for applications in different fields of interest and contribute to the expanding electroanalytical chemistry field. This is due to inherent characteristics that can be better (or only) achieved with the use of SPEs, including miniaturization, cost reduction, lower sample consumption, compatibility with portable equipment, and disposability. SPEs are also quite versatile; they can be manufactured using different formulations of conductive inks and substrates, and are of varied designs. Naturally, the analytical performance of SPEs is directly affected by the quality of the material used for printing and modifying the electrodes. In this sense, the most varied carbon nanomaterials have been explored for the preparation and modification of SPEs, providing devices with an enhanced electrochemical response and greater sensitivity, in addition to functionalized surfaces that can immobilize biological agents for the manufacture of biosensors. Considering the relevance and timeliness of the topic, this review aimed to provide an overview of the current scenario of the use of carbonaceous nanomaterials in the context of making electrochemical SPE sensors, from which different approaches will be presented, exploring materials traditionally investigated in electrochemistry, such as graphene, carbon nanotubes, carbon black, and those more recently investigated for this (carbon quantum dots, graphitic carbon nitride, and biochar). Perspectives on the use and expansion of these devices are also considered.
Keywords: biochar; carbon black; carbon nanotubes; carbon quantum dots; carbonaceous nanomaterials; disposable electrodes; electroanalysis; graphene; graphitic carbon nitride; screen-printing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
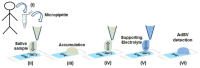
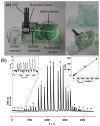


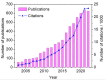

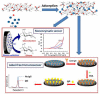





References
-
- Simões F.R., Xavier M.G. Nanoscience and Its Applications. Elsevier; Amsterdam, The Netherlands: 2017. Electrochemical Sensors; pp. 155–178.
-
- Ferrari A.G.-M., Rowley-Neale S.J., Banks C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open. 2021;3:100032. doi: 10.1016/j.talo.2021.100032. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- APQ-00083-21/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- APQ-03113-22/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- 2017/21097-3/São Paulo Research Foundation
- 301796/2022-0/National Council for Scientific and Technological Development
- Financial code 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources

