A hybrid multi-particle approach to range assessment-based treatment verification in particle therapy
- PMID: 37185591
- PMCID: PMC10130067
- DOI: 10.1038/s41598-023-33777-w
A hybrid multi-particle approach to range assessment-based treatment verification in particle therapy
Abstract
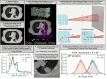
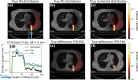
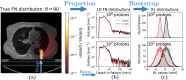
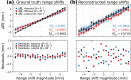
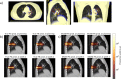
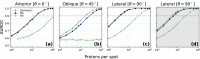
Particle therapy (PT) used for cancer treatment can spare healthy tissue and reduce treatment toxicity. However, full exploitation of the dosimetric advantages of PT is not yet possible due to range uncertainties, warranting development of range-monitoring techniques. This study proposes a novel range-monitoring technique introducing the yet unexplored concept of simultaneous detection and imaging of fast neutrons and prompt-gamma rays produced in beam-tissue interactions. A quasi-monolithic organic detector array is proposed, and its feasibility for detecting range shifts in the context of proton therapy is explored through Monte Carlo simulations of realistic patient models and detector resolution effects. The results indicate that range shifts of [Formula: see text] can be detected at relatively low proton intensities ([Formula: see text] protons/spot) when spatial information obtained through imaging of both particle species are used simultaneously. This study lays the foundation for multi-particle detection and imaging systems in the context of range verification in PT.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Jermann, M. Particle Therapy Patient Statistics (per end of 2021) (PTCOG, 2021). https://ptcog.ch.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

