FDA-approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness
- PMID: 37186121
- PMCID: PMC10600328
- DOI: 10.1002/alz.13063
FDA-approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness
Erratum in
-
Correction to "FDA-approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness".Alzheimers Dement. 2024 Mar;20(3):2297. doi: 10.1002/alz.13499. Epub 2024 Jan 31. Alzheimers Dement. 2024. PMID: 38294137 Free PMC article. No abstract available.
Abstract
Introduction: Cerebrovascular pathology is an early and causal hallmark of Alzheimer's disease (AD), in need of effective therapies.
Methods: Based on the success of our previous in vitro studies, we tested for the first time in a model of AD and cerebral amyloid angiopathy (CAA), the carbonic anhydrase inhibitors (CAIs) methazolamide and acetazolamide, Food and Drug Administration-approved against glaucoma and high-altitude sickness.
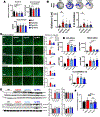
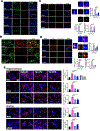
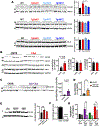
Results: Both CAIs reduced cerebral, vascular, and glial amyloid beta (Aβ) accumulation and caspase activation, diminished gliosis, and ameliorated cognition in TgSwDI mice. The CAIs also improved microvascular fitness and induced protective glial pro-clearance pathways, resulting in the reduction of Aβ deposition. Notably, we unveiled that the mitochondrial carbonic anhydrase-VB (CA-VB) is upregulated in TgSwDI brains, CAA and AD+CAA human subjects, and in endothelial cells upon Aβ treatment. Strikingly, CA-VB silencing specifically reduces Aβ-mediated endothelial apoptosis.
Discussion: This work substantiates the potential application of CAIs in clinical trials for AD and CAA.
Keywords: Alzheimer's disease; amyloid beta; astrocytes; carbonic anhydrase inhibitors; cerebral amyloid angiopathy; cerebrovascular dysfunction; clearance; endothelial cells; microglia; neuroinflammation.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Figures

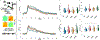


References
-
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28(4):202–208. - PubMed
-
- Ostergaard L, Aamand R, Gutierrez-Jimenez E, et al. The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging. 2013;34(4):1018–1031. - PubMed
-
- Buee L, Hof PR, Delacourte A. Brain microvascular changes in Alzheimer’s disease and other dementias. Ann N Y Acad Sci. 1997;826:7–24. - PubMed
-
- Carare RO, Aldea R, Agarwal N, et al. Clearance of interstitial fluid (ISF) and CSF (CLIC) group-part of Vascular Professional Interest Area (PIA): Cerebrovascular disease and the failure of elimination of Amyloid-beta from the brain and retina with age and Alzheimer’s disease-Opportunities for Therapy. Alzheimers Dement (Amst). 2020;12(1):e12053. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

