Evolution, persistence, and host adaption of a gonococcal AMR plasmid that emerged in the pre-antibiotic era
- PMID: 37186602
- PMCID: PMC10212123
- DOI: 10.1371/journal.pgen.1010743
Evolution, persistence, and host adaption of a gonococcal AMR plasmid that emerged in the pre-antibiotic era
Abstract
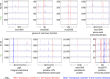
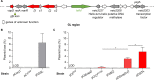
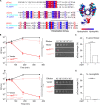
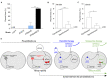
Plasmids are diverse extrachromosomal elements significantly that contribute to interspecies dissemination of antimicrobial resistance (AMR) genes. However, within clinically important bacteria, plasmids can exhibit unexpected narrow host ranges, a phenomenon that has scarcely been examined. Here we show that pConj is largely restricted to the human-specific pathogen, Neisseria gonorrhoeae. pConj can confer tetracycline resistance and is central to the dissemination of other AMR plasmids. We tracked pConj evolution from the pre-antibiotic era 80 years ago to the modern day and demonstrate that, aside from limited gene acquisition and loss events, pConj is remarkably conserved. Notably, pConj has remained prevalent in gonococcal populations despite cessation of tetracycline use, thereby demonstrating pConj adaptation to its host. Equally, pConj imposes no measurable fitness costs and is stably inherited by the gonococcus. Its maintenance depends on the co-operative activity of plasmid-encoded Toxin:Antitoxin (TA) and partitioning systems rather than host factors. An orphan VapD toxin encoded on pConj forms a split TA with antitoxins expressed from an ancestral co-resident plasmid or a horizontally-acquired chromosomal island, potentially explaining pConj's limited distribution. Finally, ciprofloxacin can induce loss of this highly stable plasmid, reflecting epidemiological evidence of transient reduction in pConj prevalence when fluoroquinolones were introduced to treat gonorrhoea.
Copyright: © 2023 Yee et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Sandegren L, Linkevicius M, Lytsy B, Melhus Å, Andersson DI. Transfer of an Escherichia coli ST131 multiresistance cassette has created a Klebsiella pneumoniae-specific plasmid associated with a major nosocomial outbreak. J Antimicrob Chemother. 2012;67: 74–83. - PubMed
-
- Rozwandowicz M, Brouwer MSM, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, et al.. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73: 1121–1137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

