Loss of insulin signaling in astrocytes exacerbates Alzheimer-like phenotypes in a 5xFAD mouse model
- PMID: 37186836
- PMCID: PMC10214134
- DOI: 10.1073/pnas.2220684120
Loss of insulin signaling in astrocytes exacerbates Alzheimer-like phenotypes in a 5xFAD mouse model
Abstract
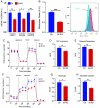
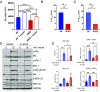
Brain insulin signaling controls peripheral energy metabolism and plays a key role in the regulation of mood and cognition. Epidemiological studies have indicated a strong connection between type 2 diabetes (T2D) and neurodegenerative disorders, especially Alzheimer's disease (AD), linked via dysregulation of insulin signaling, i.e., insulin resistance. While most studies have focused on neurons, here, we aim to understand the role of insulin signaling in astrocytes, a glial cell type highly implicated in AD pathology and AD progression. To this end, we created a mouse model by crossing 5xFAD transgenic mice, a well-recognized AD mouse model that expresses five familial AD mutations, with mice carrying a selective, inducible insulin receptor (IR) knockout in astrocytes (iGIRKO). We show that by age 6 mo, iGIRKO/5xFAD mice exhibited greater alterations in nesting, Y-maze performance, and fear response than those of mice with the 5xFAD transgenes alone. This was associated with increased Tau (T231) phosphorylation, increased Aβ plaque size, and increased association of astrocytes with plaques in the cerebral cortex as assessed using tissue CLARITY of the brain in the iGIRKO/5xFAD mice. Mechanistically, in vitro knockout of IR in primary astrocytes resulted in loss of insulin signaling, reduced ATP production and glycolic capacity, and impaired Aβ uptake both in the basal and insulin-stimulated states. Thus, insulin signaling in astrocytes plays an important role in the control of Aβ uptake, thereby contributing to AD pathology, and highlighting the potential importance of targeting insulin signaling in astrocytes as a site for therapeutics for patients with T2D and AD.
Keywords: Alzheimer’s disease; astrocytes; diabetes; insulin resistance; neurons.
Conflict of interest statement
The lead author, C.R.K., and one reviewer, D.A., are co-authors of a recent publication (DOI:
Figures



References
-
- Kahn C. R., Suzuki R., “Insulin action in the brain and the pathogenesis of alzheimer’s disease” in Diabetes, Insulin and Alzheimer’s Disease, Craft S., Christen Y., Eds. (Springer, Berlin Heidelberg, Berlin, Heidelberg, 2010), pp. 1–20, 10.1007/978-3-642-04300-0_1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

