SMARCB1 regulates the hypoxic stress response in sickle cell trait
- PMID: 37186844
- PMCID: PMC10214195
- DOI: 10.1073/pnas.2209639120
SMARCB1 regulates the hypoxic stress response in sickle cell trait
Abstract
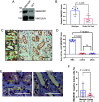
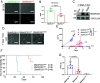
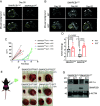
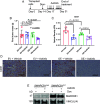
Renal medullary carcinoma (RMC) is an aggressive kidney cancer that almost exclusively develops in individuals with sickle cell trait (SCT) and is always characterized by loss of the tumor suppressor SMARCB1. Because renal ischemia induced by red blood cell sickling exacerbates chronic renal medullary hypoxia in vivo, we investigated whether the loss of SMARCB1 confers a survival advantage under the setting of SCT. Hypoxic stress, which naturally occurs within the renal medulla, is elevated under the setting of SCT. Our findings showed that hypoxia-induced SMARCB1 degradation protected renal cells from hypoxic stress. SMARCB1 wild-type renal tumors exhibited lower levels of SMARCB1 and more aggressive growth in mice harboring the SCT mutation in human hemoglobin A (HbA) than in control mice harboring wild-type human HbA. Consistent with established clinical observations, SMARCB1-null renal tumors were refractory to hypoxia-inducing therapeutic inhibition of angiogenesis. Further, reconstitution of SMARCB1 restored renal tumor sensitivity to hypoxic stress in vitro and in vivo. Together, our results demonstrate a physiological role for SMARCB1 degradation in response to hypoxic stress, connect the renal medullary hypoxia induced by SCT with an increased risk of SMARCB1-negative RMC, and shed light into the mechanisms mediating the resistance of SMARCB1-null renal tumors against angiogenesis inhibition therapies.
Keywords: SMARCB1; hypoxia; renal medullary carcinoma; sickle cell trait.
Conflict of interest statement
Pavlos Msaouel has received honoraria for service on a Scientific Advisory Board for Mirati Therapeutics, Bristol Myers Squibb, and Exelixis; consulting for Axiom Healthcare Strategies; non-branded educational programs supported by Exelixis and Pfizer; and research funding for clinical trials from Takeda, Bristol Myers Squibb, Mirati Therapeutics, Gateway for Cancer Research, and UT MD Anderson Cancer Center.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

