Mechanisms underlying the efficacy of a rodent model of vertical sleeve gastrectomy - A focus on energy expenditure
- PMID: 37187239
- PMCID: PMC10250165
- DOI: 10.1016/j.molmet.2023.101739
Mechanisms underlying the efficacy of a rodent model of vertical sleeve gastrectomy - A focus on energy expenditure
Abstract
Objective: Bariatric surgery remains the only effective and durable treatment option for morbid obesity. Vertical Sleeve Gastrectomy (VSG) is currently the most widely performed of these surgeries primarily because of its proven efficacy in generating rapid onset weight loss, improved glucose regulation and reduced mortality compared with other invasive procedures. VSG is associated with reduced appetite, however, the relative importance of energy expenditure to VSG-induced weight loss and changes in glucose regulation, particularly that in brown adipose tissue (BAT), remains unclear. The aim of this study was to investigate the role of BAT thermogenesis in the efficacy of VSG in a rodent model.
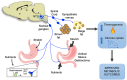
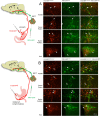
Methods: Diet-induced obese male Sprague-Dawley rats were either sham-operated, underwent VSG surgery or were pair-fed to the food consumed by the VSG group. Rats were also implanted with biotelemetry devices between the interscapular lobes of BAT to assess local changes in BAT temperature as a surrogate measure of thermogenic activity. Metabolic parameters including food intake, body weight and changes in body composition were assessed. To further elucidate the contribution of energy expenditure via BAT thermogenesis to VSG-induced weight loss, a separate cohort of chow-fed rats underwent complete excision of the interscapular BAT (iBAT lipectomy) or chemical denervation using 6-hydroxydopamine (6-OHDA). To localize glucose uptake in specific tissues, an oral glucose tolerance test was combined with an intraperitoneal injection of 14C-2-deoxy-d-glucose (14C-2DG). Transneuronal viral tracing was used to identify 1) sensory neurons directed to the stomach or small intestine (H129-RFP) or 2) chains of polysynaptically linked neurons directed to BAT (PRV-GFP) in the same animals.
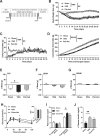
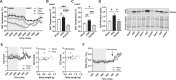
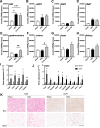
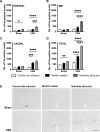
Results: Following VSG, there was a rapid reduction in body weight that was associated with reduced food intake, elevated BAT temperature and improved glucose regulation. Rats that underwent VSG had elevated glucose uptake into BAT compared to sham operated animals as well as elevated gene markers related to increased BAT activity (Ucp1, Dio2, Cpt1b, Cox8b, Ppargc) and markers of increased browning of white fat (Ucp1, Dio2, Cited1, Tbx1, Tnfrs9). Both iBAT lipectomy and 6-OHDA treatment significantly attenuated the impact of VSG on changes in body weight and adiposity in chow-fed animals. In addition, surgical excision of iBAT following VSG significantly reversed VSG-mediated improvements in glucose tolerance, an effect that was independent of circulating insulin levels. Viral tracing studies highlighted a patent neural link between the gut and BAT that included groups of premotor BAT-directed neurons in the dorsal raphe and raphe pallidus.
Conclusions: Collectively, these data support a role for BAT in mediating the metabolic sequelae following VSG surgery, particularly the improvement in glucose regulation, and highlight the need to better understand the contribution from this tissue in human patients.
Keywords: Animal model; Bariatric surgery; Brown adipose tissue thermogenesis; Energy expenditure; Metabolic surgery; Vertical sleeve gastrectomy.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: WAB reports grants from Johnson and Johnson, Medtronic, GORE, Applied Medical, Novo Nordisk, Myerton and the Australian Commonwealth Government and personal fees from GORE, Novo Nordisk, Pfizer and Merck Sharpe and Dohme for lectures and advisory boards.
Figures


References
-
- Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. - PubMed
-
- O'Brien P.E., Hindle A., Brennan L., Skinner S., Burton P., Smith A., et al. Long-Term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3–14. - PMC - PubMed
-
- Salminen P., Helmiö M., Ovaska J., Juuti A., Leivonen M., Peromaa-Haavisto P., et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 Years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. Jama. 2018;319(3):241–254. - PMC - PubMed

