Neutrophil elastase decreases SARS-CoV-2 spike protein binding to human bronchial epithelia by clipping ACE-2 ectodomain from the epithelial surface
- PMID: 37187291
- PMCID: PMC10181948
- DOI: 10.1016/j.jbc.2023.104820
Neutrophil elastase decreases SARS-CoV-2 spike protein binding to human bronchial epithelia by clipping ACE-2 ectodomain from the epithelial surface
Abstract
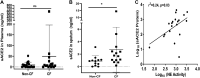
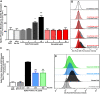
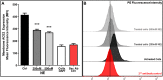
Patients with cystic fibrosis (CF) have decreased severity of severe acute respiratory syndrome-like coronavirus-2 (SARS-CoV-2) infections, but the underlying cause is unknown. Patients with CF have high levels of neutrophil elastase (NE) in the airway. We examined whether respiratory epithelial angiotensin-converting enzyme 2 (ACE-2), the receptor for the SARS-CoV-2 spike protein, is a proteolytic target of NE. Soluble ACE-2 levels were quantified by ELISA in airway secretions and serum from patients with and without CF, the association between soluble ACE-2 and NE activity levels was evaluated in CF sputum. We determined that NE activity was directly correlated with increased ACE-2 in CF sputum. Additionally, primary human bronchial epithelial (HBE) cells, exposed to NE or control vehicle, were evaluated by Western analysis for the release of cleaved ACE-2 ectodomain fragment into conditioned media, flow cytometry for the loss of cell surface ACE-2, its impact on SARS-CoV-2 spike protein binding. We found that NE treatment released ACE-2 ectodomain fragment from HBE and decreased spike protein binding to HBE. Furthermore, we performed NE treatment of recombinant ACE-2-Fc-tagged protein in vitro to assess whether NE was sufficient to cleave recombinant ACE-2-Fc protein. Proteomic analysis identified specific NE cleavage sites in the ACE-2 ectodomain that would result in loss of the putative N-terminal spike-binding domain. Collectively, data support that NE plays a disruptive role in SARS-CoV-2 infection by catalyzing ACE-2 ectodomain shedding from the airway epithelia. This mechanism may reduce SARS-CoV-2 virus binding to respiratory epithelial cells and decrease the severity of COVID19 infection.
Keywords: ACE-2; SARS-CoV-2; airway epithelial cell; ectodomain cleavage; mass spectrometry; neutrophil; proteinase; spike protein; virus entry.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Voynow J.A., Mascarenhas M., Kelly A., Scanlin T.F. In: Fishman's Pulmonary Disesases and Disorders. 5th Ed. Grippi M.A., Elias J.A., Fishman J.A., Kotloff R.M., Pack A.I., Senior R.M., et al., editors. McGraw-Hill Education; New York, NY: 2015. Cystic fibrosis; pp. 757–778.
-
- Zheng S., De B.P., Choudhary S., Comhair S.A., Goggans T., Slee R., et al. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003;18:619–630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

