The NSs protein of severe fever with thrombocytopenia syndrome virus differentially inhibits the type 1 interferon response among animal species
- PMID: 37187292
- PMCID: PMC10276162
- DOI: 10.1016/j.jbc.2023.104819
The NSs protein of severe fever with thrombocytopenia syndrome virus differentially inhibits the type 1 interferon response among animal species
Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV), which has been reported in China, Korea, Japan, Vietnam, and Taiwan, is a causative agent of severe fever thrombocytopenia syndrome. This virus has a high mortality and induces thrombocytopenia and leukocytopenia in humans, cats, and aged ferrets, whereas immunocompetent adult mice infected with SFTSV never show symptoms. Anti-SFTSV antibodies have been detected in several animals-including goats, sheep, cattle, and pigs. However, there are no reports of severe fever thrombocytopenia syndrome in these animals. Previous studies have reported that the nonstructural protein NSs of SFTSV inhibits the type I interferon (IFN-I) response through the sequestration of human signal transducer and activator of transcription (STAT) proteins. In this study, comparative analysis of the function of NSs as IFN antagonists in human, cat, dog, ferret, mouse, and pig cells revealed a correlation between pathogenicity of SFTSV and the function of NSs in each animal. Furthermore, we found that the inhibition of IFN-I signaling and phosphorylation of STAT1 and STAT2 by NSs depended on the binding ability of NSs to STAT1 and STAT2. Our results imply that the function of NSs in antagonizing STAT2 determines the species-specific pathogenicity of SFTSV.
Keywords: NSs; SFTSV; STAT2; animal; animal virus; innate immunity; interferon; negative-strand RNA virus; signal transducers and activators of transcription 1.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

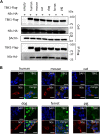
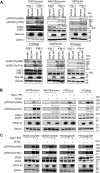
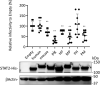
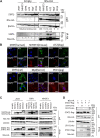
 ) represent SFTSV WT and SFTSV ΔNSs, respectively. Values are the averages with SDs of data from three independent experiments (n = 3). ∗p < 0.05 and ∗∗p < 0.01, SFTSV WT infected versus SFTSV ΔNSs infected. Each exact p value, average, and SD is shown in Table S1. CRFK, Crandell–Rees feline kidney; HEK293T, human embryonic kidney 293T cell line; MOI, multiplicity of infection; ND, not detected; NSs, nonstructural protein; SFTSV, severe fever with thrombocytopenia syndrome virus.
) represent SFTSV WT and SFTSV ΔNSs, respectively. Values are the averages with SDs of data from three independent experiments (n = 3). ∗p < 0.05 and ∗∗p < 0.01, SFTSV WT infected versus SFTSV ΔNSs infected. Each exact p value, average, and SD is shown in Table S1. CRFK, Crandell–Rees feline kidney; HEK293T, human embryonic kidney 293T cell line; MOI, multiplicity of infection; ND, not detected; NSs, nonstructural protein; SFTSV, severe fever with thrombocytopenia syndrome virus.




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

