Bioinformatics Analysis to Uncover the Potential Drug Targets Responsible for Mycobacterium tuberculosis Peptidoglycan and Lysine Biosynthesis
- PMID: 37187890
- PMCID: PMC10176782
- DOI: 10.1177/11779322231171774
Bioinformatics Analysis to Uncover the Potential Drug Targets Responsible for Mycobacterium tuberculosis Peptidoglycan and Lysine Biosynthesis
Abstract
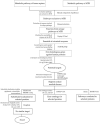
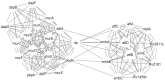
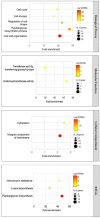
Drug-resistant tuberculosis (TB), which results mainly from the selection of naturally resistant strains of Mycobacterium tuberculosis (MTB) due to mismanaged treatment, poses a severe challenge to the global control of TB. Therefore, screening novel and unique drug targets against this pathogen is urgently needed. The metabolic pathways of Homo sapiens and MTB were compared using the Kyoto Encyclopedia of Genes and Genomes tool, and further, the proteins that are involved in the metabolic pathways of MTB were subtracted and proceeded to protein-protein interaction network analysis, subcellular localization, drug ability testing, and gene ontology. The study aims to identify enzymes for the unique pathways for further screening to determine the feasibility of the therapeutic targets. The qualitative characteristics of 28 proteins identified as drug target candidates were studied. The results showed that 12 were cytoplasmic, 2 were extracellular, 12 were transmembrane, and 3 were unknown. Furthermore, druggability analysis revealed 14 druggable proteins, of which 12 were novel and responsible for MTB peptidoglycan and lysine biosynthesis. The novel targets obtained in this study are used to develop antimicrobial treatments against pathogenic bacteria. Future studies should further shed light on the clinical implementation to identify antimicrobial therapies against MTB.
Keywords: In silico drug targets; Mycobacterium tuberculosis; metabolic pathways; protein-protein interaction.
© The Author(s) 2023.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Editorial: Current status and perspective on drug targets in tubercle bacilli and drug design of antituberculous agents based on structure-activity relationship.Curr Pharm Des. 2014;20(27):4305-6. doi: 10.2174/1381612819666131118203915. Curr Pharm Des. 2014. PMID: 24245755
-
Identification of glucosyl-3-phosphoglycerate phosphatase as a novel drug target against resistant strain of Mycobacterium tuberculosis (XDR1219) by using comparative metabolic pathway approach.Comput Biol Chem. 2019 Apr;79:91-102. doi: 10.1016/j.compbiolchem.2019.01.011. Epub 2019 Jan 24. Comput Biol Chem. 2019. PMID: 30743161
-
Mycobacterium tuberculosis H37Rv: In Silico Drug Targets Identification by Metabolic Pathways Analysis.Int J Evol Biol. 2014;2014:284170. doi: 10.1155/2014/284170. Epub 2014 Feb 25. Int J Evol Biol. 2014. PMID: 24719775 Free PMC article.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Revisiting Anti-tuberculosis Therapeutic Strategies That Target the Peptidoglycan Structure and Synthesis.Front Microbiol. 2019 Feb 11;10:190. doi: 10.3389/fmicb.2019.00190. eCollection 2019. Front Microbiol. 2019. PMID: 30804921 Free PMC article. Review.
Cited by
-
Cold stress enhances cryotolerance in Lacticaseibacillus rhamnosus B6 via membrane lipid remodeling and differential protein expression.Curr Res Microb Sci. 2025 Aug 5;9:100453. doi: 10.1016/j.crmicr.2025.100453. eCollection 2025. Curr Res Microb Sci. 2025. PMID: 40837524 Free PMC article.
-
Exploring optimal drug targets through subtractive proteomics analysis and pangenomic insights for tailored drug design in tuberculosis.Sci Rep. 2024 May 13;14(1):10904. doi: 10.1038/s41598-024-61752-6. Sci Rep. 2024. PMID: 38740859 Free PMC article.
-
Amino Acid Biosynthesis Inhibitors in Tuberculosis Drug Discovery.Pharmaceutics. 2024 May 28;16(6):725. doi: 10.3390/pharmaceutics16060725. Pharmaceutics. 2024. PMID: 38931847 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

