Neonatal subarachnoid hemorrhage disrupts multiple aspects of cerebellar development
- PMID: 37187957
- PMCID: PMC10175619
- DOI: 10.3389/fnmol.2023.1161086
Neonatal subarachnoid hemorrhage disrupts multiple aspects of cerebellar development
Abstract
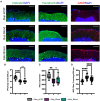
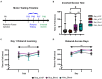
Over the past decade, survival rates for extremely low gestational age neonates (ELGANs; <28 weeks gestation) has markedly improved. Unfortunately, a significant proportion of ELGANs will suffer from neurodevelopmental dysfunction. Cerebellar hemorrhagic injury (CHI) has been increasingly recognized in the ELGANs population and may contribute to neurologic dysfunction; however, the underlying mechanisms are poorly understood. To address this gap in knowledge, we developed a novel model of early isolated posterior fossa subarachnoid hemorrhage (SAH) in neonatal mice and investigated both acute and long-term effects. Following SAH on postnatal day 6 (P6), we found significant decreased levels of proliferation with the external granular layer (EGL), thinning of the EGL, decreased Purkinje cell (PC) density, and increased Bergmann glial (BG) fiber crossings at P8. At P42, CHI resulted in decreased PC density, decreased molecular layer interneuron (MLI) density, and increased BG fiber crossings. Results from both Rotarod and inverted screen assays did not demonstrate significant effects on motor strength or learning at P35-38. Treatment with the anti-inflammatory drug Ketoprofen did not significantly alter our findings after CHI, suggesting that treatment of neuro-inflammation does not provide significant neuroprotection post CHI. Further studies are required to fully elucidate the mechanisms through which CHI disrupts cerebellar developmental programming in order to develop therapeutic strategies for neuroprotection in ELGANs.
Keywords: Purkinje cells; cerebellar development; cerebellar granule cells; cerebellar hemorrhage; preterm brain injury.
Copyright © 2023 Butler, Skibo, Traudt and Millen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Update of
-
Neonatal Subarachnoid Hemorrhage Disrupts Multiple Aspects of Cerebellar Development.bioRxiv [Preprint]. 2023 Feb 10:2023.02.10.528048. doi: 10.1101/2023.02.10.528048. bioRxiv. 2023. Update in: Front Mol Neurosci. 2023 Apr 28;16:1161086. doi: 10.3389/fnmol.2023.1161086. PMID: 36798230 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

