COF-300 synthesis and colloidal stabilization with substituted benzoic acids
- PMID: 37188250
- PMCID: PMC10176042
- DOI: 10.1039/d3ra02202a
COF-300 synthesis and colloidal stabilization with substituted benzoic acids
Abstract
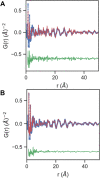
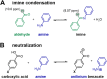
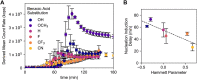
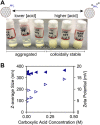
Colloidal covalent organic framework (COF) synthesis enables morphological control of crystallite size and shape. Despite numerous examples of 2D COF colloids with various linkage chemistries, 3D imine-linked COF colloids are more challenging synthetic targets. Here we report a rapid (15 min-5 day) synthesis of hydrated COF-300 colloids ranging in length (251 nm-4.6 μm) with high crystallinity and moderate surface areas (150 m2 g-1). These materials are characterized by pair distribution function analysis, which is consistent with the known average structure for this material alongside different degrees of atomic disorder at different length scales. Additionally, we investigate a series of para-substituted benzoic acid catalysts, finding that 4-cyano and 4-fluoro substituted benzoic acids produce the largest COF-300 crystallites with lengths of 1-2 μm. In situ dynamic light scattering experiments are used to assess time to nucleation in conjunction with 1H NMR model compound studies to probe the impact of catalyst acidity on the imine condensation equilibrium. We observe cationically stabilized colloids with a zeta potential of up to +14.35 mV in benzonitrile as a result of the carboxylic acid catalyst protonating surface amine groups. We leverage these surface chemistry insights to synthesize small COF-300 colloids using sterically hindered diortho-substituted carboxylic acid catalysts. This fundamental study of COF-300 colloid synthesis and surface chemistry will provide new insights into the role of acid catalysts both as imine condensation catalysts and as colloid stabilizing agents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Ma T. Kapustin E. A. Yin S. X. Liang L. Zhou Z. Niu J. Li L.-H. Wang Y. Su J. Li J. Wang X. Wang W. D. Wang W. Sun J. Yaghi O. M. Science. 2018;361:48–52. - PubMed
-
- Feng X. Ding X. Jiang D. Chem. Soc. Rev. 2012;41:6010–6022. - PubMed
-
- Tan K. T. Ghosh S. Wang Z. Wen F. Rodríguez-San-Miguel D. Feng J. Huang N. Wang W. Zamora F. Feng X. Nat. Rev. Methods Primers. 2023;3:1–19.
-
- Zeng Y. Zou R. Zhao Y. Adv. Mater. 2016;28:2855–2873. - PubMed
-
- Lin S. Diercks C. S. Zhang Y.-B. Kornienko N. Nichols E. M. Zhao Y. Paris A. R. Kim D. Yang P. Yaghi O. M. Science. 2015;349:1208–1213. - PubMed
LinkOut - more resources
Full Text Sources

