Glutamatergic cerebellar neurons differentially contribute to the acquisition of motor and social behaviors
- PMID: 37188723
- PMCID: PMC10185563
- DOI: 10.1038/s41467-023-38475-9
Glutamatergic cerebellar neurons differentially contribute to the acquisition of motor and social behaviors
Abstract
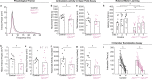
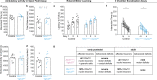
Insults to the developing cerebellum can cause motor, language, and social deficits. Here, we investigate whether developmental insults to different cerebellar neurons constrain the ability to acquire cerebellar-dependent behaviors. We perturb cerebellar cortical or nuclei neuron function by eliminating glutamatergic neurotransmission during development, and then we measure motor and social behaviors in early postnatal and adult mice. Altering cortical and nuclei neurons impacts postnatal motor control and social vocalizations. Normalizing neurotransmission in cortical neurons but not nuclei neurons restores social behaviors while the motor deficits remain impaired in adults. In contrast, manipulating only a subset of nuclei neurons leaves social behaviors intact but leads to early motor deficits that are restored by adulthood. Our data uncover that glutamatergic neurotransmission from cerebellar cortical and nuclei neurons differentially control the acquisition of motor and social behaviors, and that the brain can compensate for some but not all perturbations to the developing cerebellum.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


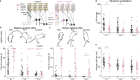





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

