Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial
- PMID: 37188783
- PMCID: PMC10287560
- DOI: 10.1038/s41591-023-02347-y
Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial
Erratum in
-
Author Correction: Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial.Nat Med. 2025 Sep;31(9):3204. doi: 10.1038/s41591-025-03895-1. Nat Med. 2025. PMID: 40691367 Free PMC article. No abstract available.
Abstract
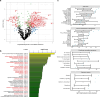
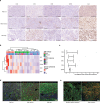
Immune-mediated anti-tumoral responses, elicited by oncolytic viruses and augmented with checkpoint inhibition, may be an effective treatment approach for glioblastoma. Here in this multicenter phase 1/2 study we evaluated the combination of intratumoral delivery of oncolytic virus DNX-2401 followed by intravenous anti-PD-1 antibody pembrolizumab in recurrent glioblastoma, first in a dose-escalation and then in a dose-expansion phase, in 49 patients. The primary endpoints were overall safety and objective response rate. The primary safety endpoint was met, whereas the primary efficacy endpoint was not met. There were no dose-limiting toxicities, and full dose combined treatment was well tolerated. The objective response rate was 10.4% (90% confidence interval (CI) 4.2-20.7%), which was not statistically greater than the prespecified control rate of 5%. The secondary endpoint of overall survival at 12 months was 52.7% (95% CI 40.1-69.2%), which was statistically greater than the prespecified control rate of 20%. Median overall survival was 12.5 months (10.7-13.5 months). Objective responses led to longer survival (hazard ratio 0.20, 95% CI 0.05-0.87). A total of 56.2% (95% CI 41.1-70.5%) of patients had a clinical benefit defined as stable disease or better. Three patients completed treatment with durable responses and remain alive at 45, 48 and 60 months. Exploratory mutational, gene-expression and immunophenotypic analyses revealed that the balance between immune cell infiltration and expression of checkpoint inhibitors may potentially inform on response to treatment and mechanisms of resistance. Overall, the combination of intratumoral DNX-2401 followed by pembrolizumab was safe with notable survival benefit in select patients (ClinicalTrials.gov registration: NCT02798406).
© 2023. The Author(s).
Conflict of interest statement
A.M.S. has received in-kind (drug) support from BMS, in-kind (ultrasound devices) and research support from Carthera, and in-kind (drug) and research support from Agenus. A.R. is a Medexus consultant (paid), on the Nico Oncology Tissue Advisory Board (unpaid), and has an extramural grant from the Bristol Myers Squibb Diversity in Clinical Trials Program. C.C.C. is a consultant for Clearpoint Neuro and Medtronic, and receives travel reimbursement from GTMedical for lectures. C.G.-M. has license agreements with DNATrix, and is a shareholder of DNATrix. F.F.L. is a patent holder for DNX-2401. H.C. is on the advisory/consult for Best Doctors/Teladoc, Orbus Therapeutics, Bristol Meyers Squibb, Regeneron, Novocure Research Funding (Site PI/Institutional Contract): Newlink Genetics, Plexxikon, Kadmon, Orbus, Merck, DNATrix, Abbvie, Beigene, Forma Therapeutics, GCAR, Array BioPharma, Karyopharm Therapeutics, Nuvation Bio, Bayer, Bristol Meyers Squibb, Sumitomo Dainippon Pharma Oncology, Samus Therapeutics and Erasca. J.F. has license agreements with DNATrix, and is a shareholder of DNATrix. J.M.M. has been a speaker for AstraZeneca, Janssen and TG Therapeutics. J.R. is employed by DNATrix. M.A.V. is consulting/receives honoraria from Chimerix, Midatech and Olympus, their hospital has contracts with Infuseon, Oncosynergy, Celgene, Denovo, Chimerix and NIH, and has patent rights for Cleveland Clinic/Infuseon. M.M.A. has a research grant from DNATrix unrelated to this work. P.U.K. receives consulting fees from Enclear Therapies, Affinia Therapeutics, Biocept, Janssen, Sintetica, Bioclinica/Clario, Novocure, Mirati and Orbus Therapeutics, performs contracted research for Genentech, Novocure, DNAtrix and Orbus Therapeutics, is a scientific consultant for Enclear Therapies (with grant options provided) and has intellectual properties without financial gain of European Patent 3307327, 12 August 2020 and US Patent Pending 15/737,188. R.A. is a consultant for Tactical Therapeutics and Axelar AB. R.S. is a consultant and receives fees from Alpheus Medical, AstraZeneca, Carthera, Celularity, GT Medical, Insightec, Black Diamond, Northwest Therapeutics, Syneos Health (Boston Biomedical) and Varian Medical Systems, is a consultant and their institution receives fees from Boston Scientific, is a consultant and receives no fees from Novocure, is an IP holder for therapeutic ultrasound indications and use, is a Board Member and former president of the European Organisation for Research Treatment of Cancer, and has stock options in Alpheus Medical and Carthera. S.G.N. is an Institutional PI of multicenter trials with BMS, Merck, Strata Oncology and Mirati Therapeutics, and is the Chair of ABIM Med Onc Board. S.K. receives honorarium from Novocure, is reimbursed/has sponsored travel from Novocure, is consulting for Pacific Marine Biotech, Autem Therapeutics, and is management/holds a director position for SKBio Advisory, LLC. T.F.C. is cofounder, major stock holder, consultant and board member of Katmai Pharmaceuticals, holds stock for Erasca, is member of the board and paid consultant for the 501c3 Global Coalition for Adaptive Research, holds stock in Chimerix and receives milestone payments and possible future royalties, is member of the scientific advisory board for Break Through Cancer, is member of the scientific advisory board for Cure Brain Cancer Foundation, and has provided paid consulting services to Blue Rock, Vida Ventures, Lista Therapeutics, Stemline, Novartis, Roche, Sonalasense, Sagimet, Clinical Care Options, Ideology Health, Servier, Jubilant, Immvira, Gan & Lee, BrainStorm, Katmai, Sapience, Inovio, Vigeo Therapeutics, DNATrix, Tyme, SDP, Kintara, Bayer, Merck, Boehinger Ingelheim, VBL, Amgen, Kiyatec, Odonate Therapeutics QED, Medefield, Pascal Biosciences, Bayer, Tocagen, Karyopharm, GW Pharma, Abbvie, VBI, Deciphera, VBL, Agios, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Novocure, Novogen, Boston Biomedical, Sunovion, Insys, Pfizer, Notable labs, Medqia, Trizel, Medscape and has contracts with UCLA for the Brain Tumor Program with Roche, VBI, Merck, Novartis, BMS, AstraZeneca and Servier. The Regents of the University of California (T.F.C. employer) has licensed intellectual property co-invented by T.F.C. to Katmai Pharmaceuticals. V.K.P. receives clinical trial support from Servier, Karyopharm, Samus Therapeutics and Radiomedix, receives research support from Karyopharm, Bexion, SK Lifesciences and J INTS Bio, is a consultant for Servier, Insightec, Novocure, NewBio and Orbus Therapeutics, and has equity in Gilead and Amarin. W.P.M. is a consultant for Century Therapeutics, Ono Therapeutics and Novocure, and receives research funding from Hoffman La Roche, Agios and Orbus Therapeutics. The remaining authors declare no competing interests.
Figures







References
-
- Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med.352, 987–996 (2005). - PubMed
-
- Taal, W. et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol.15, 943–953 (2014). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

