Author Correction: Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment
- PMID: 37188807
- PMCID: PMC10229603
- DOI: 10.1038/s41423-023-01010-3
Author Correction: Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment
Figures
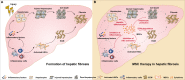
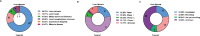
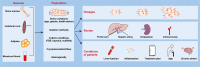

Erratum for
-
Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment.Cell Mol Immunol. 2023 Jun;20(6):583-599. doi: 10.1038/s41423-023-00983-5. Epub 2023 Feb 24. Cell Mol Immunol. 2023. PMID: 36823236 Free PMC article. Review.
Publication types
LinkOut - more resources
Full Text Sources

