Ticks harbor and excrete chronic wasting disease prions
- PMID: 37188858
- PMCID: PMC10185559
- DOI: 10.1038/s41598-023-34308-3
Ticks harbor and excrete chronic wasting disease prions
Abstract
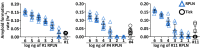
Chronic wasting disease (CWD) is a fatal neurodegenerative disease caused by infectious prions (PrPCWD) affecting cervids. Circulating PrPCWD in blood may pose a risk for indirect transmission by way of hematophagous ectoparasites acting as mechanical vectors. Cervids can carry high tick infestations and exhibit allogrooming, a common tick defense strategy between conspecifics. Ingestion of ticks during allogrooming may expose naïve animals to CWD, if ticks harbor PrPCWD. This study investigates whether ticks can harbor transmission-relevant quantities of PrPCWD by combining experimental tick feeding trials and evaluation of ticks from free-ranging white-tailed deer (Odocoileus virginianus). Using the real-time quaking-induced conversion (RT-QuIC) assay, we show that black-legged ticks (Ixodes scapularis) fed PrPCWD-spiked blood using artificial membranes ingest and excrete PrPCWD. Combining results of RT-QuIC and protein misfolding cyclic amplification, we detected seeding activity from 6 of 15 (40%) pooled tick samples collected from wild CWD-infected white-tailed deer. Seeding activities in ticks were analogous to 10-1000 ng of CWD-positive retropharyngeal lymph node collected from deer upon which they were feeding. Estimates revealed a median infectious dose range of 0.3-42.4 per tick, suggesting that ticks can take up transmission-relevant amounts of PrPCWD and may pose a CWD risk to cervids.
© 2023. The Author(s).
Conflict of interest statement
R.M. is listed as an inventor in one patent related to the PMCA technology. H.N.I, F.B.R., S.S.L, J.A.P, D.J.S., D.P.W., and W.C.T. have no conflicts to declare for this study.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

