Lin28a maintains a subset of adult muscle stem cells in an embryonic-like state
- PMID: 37188880
- PMCID: PMC10474071
- DOI: 10.1038/s41422-023-00818-y
Lin28a maintains a subset of adult muscle stem cells in an embryonic-like state
Abstract
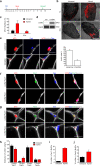
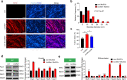
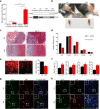
During homeostasis and after injury, adult muscle stem cells (MuSCs) activate to mediate muscle regeneration. However, much remains unclear regarding the heterogeneous capacity of MuSCs for self-renewal and regeneration. Here, we show that Lin28a is expressed in embryonic limb bud muscle progenitors, and that a rare reserve subset of Lin28a+Pax7- skeletal MuSCs can respond to injury at adult stage by replenishing the Pax7+ MuSC pool to drive muscle regeneration. Compared with adult Pax7+ MuSCs, Lin28a+ MuSCs displayed enhanced myogenic potency in vitro and in vivo upon transplantation. The epigenome of adult Lin28a+ MuSCs showed resemblance to embryonic muscle progenitors. In addition, RNA-sequencing revealed that Lin28a+ MuSCs co-expressed higher levels of certain embryonic limb bud transcription factors, telomerase components and the p53 inhibitor Mdm4, and lower levels of myogenic differentiation markers compared to adult Pax7+ MuSCs, resulting in enhanced self-renewal and stress-response signatures. Functionally, conditional ablation and induction of Lin28a+ MuSCs in adult mice revealed that these cells are necessary and sufficient for efficient muscle regeneration. Together, our findings connect the embryonic factor Lin28a to adult stem cell self-renewal and juvenile regeneration.
© 2023. The Author(s) under exclusive licence to Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

