Clinically stable covid-19 patients presenting to acute unscheduled episodic care venues have increased risk of hospitalization: secondary analysis of a randomized control trial
- PMID: 37189091
- PMCID: PMC10184108
- DOI: 10.1186/s12879-023-08295-9
Clinically stable covid-19 patients presenting to acute unscheduled episodic care venues have increased risk of hospitalization: secondary analysis of a randomized control trial
Abstract
Background: Assessment for risks associated with acute stable COVID-19 is important to optimize clinical trial enrollment and target patients for scarce therapeutics. To assess whether healthcare system engagement location is an independent predictor of outcomes we performed a secondary analysis of the ACTIV-4B Outpatient Thrombosis Prevention trial.
Methods: A secondary analysis of the ACTIV-4B trial that was conducted at 52 US sites between September 2020 and August 2021. Participants were enrolled through acute unscheduled episodic care (AUEC) enrollment location (emergency department, or urgent care clinic visit) compared to minimal contact (MC) enrollment (electronic contact from test center lists of positive patients).We report the primary composite outcome of cardiopulmonary hospitalizations, symptomatic venous thromboembolism, myocardial infarction, stroke, transient ischemic attack, systemic arterial thromboembolism, or death among stable outpatients stratified by enrollment setting, AUEC versus MC. A propensity score for AUEC enrollment was created, and Cox proportional hazards regression with inverse probability weighting (IPW) was used to compare the primary outcome by enrollment location.
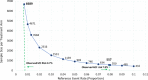
Results: Among the 657 ACTIV-4B patients randomized, 533 (81.1%) with known enrollment setting data were included in this analysis, 227 from AUEC settings and 306 from MC settings. In a multivariate logistic regression model, time from COVID test, age, Black race, Hispanic ethnicity, and body mass index were associated with AUEC enrollment. Irrespective of trial treatment allocation, patients enrolled at an AUEC setting were 10-times more likely to suffer from the adjudicated primary outcome, 7.9% vs. 0.7%; p < 0.001, compared with patients enrolled at a MC setting. Upon Cox regression analysis adjustment patients enrolled at an AUEC setting remained at significant risk of the primary composite outcome, HR 3.40 (95% CI 1.46, 7.94).
Conclusions: Patients with clinically stable COVID-19 presenting to an AUEC enrollment setting represent a population at increased risk of arterial and venous thrombosis complications, hospitalization for cardiopulmonary events, or death, when adjusted for other risk factors, compared with patients enrolled at a MC setting. Future outpatient therapeutic trials and clinical therapeutic delivery programs of clinically stable COVID-19 patients may focus on inclusion of higher-risk patient populations from AUEC engagement locations.
Trial registration: ClinicalTrials.gov Identifier: NCT04498273.
Keywords: COVID-19; CVA; Clinical trial enrollment; PE; Pulmonary embolism; SARS-CoV-2; Stroke; VTE; Venous thromboembolic disease.
© 2023. The Author(s).
Conflict of interest statement
JRB reported receiving grants payable his institution from the National Institutes of Health (NIH) for clinical trial work and receiving consulting fees from JAJ LLC. JMC reported receiving personal fees from Bristol Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi and that his institution has received research funding from CSL Behring. MB reported receiving personal fees for data and safety monitoring board membership from Cerus Corporation. JAK reported receiving consulting fees from GlaxoSmithKline and research funding from Sergey Brin Family Foundation, National Institutes of Health, American Lung Association, and the Patient Centered Outcomes Research Institute. BK reported receiving grants from SOCAR Research SA. PH reported receiving grants from Brigham and Women’s Hospital, NIH, Novartis, and CalciMedica. PMR reported receiving grants from Bristol Myers Squibb and Pfizer and serving as a consultant for work unrelated to this study for Corvidia, Novartis, Flame, Agepha, Inflazome, AstraZeneca, Janssen, Civi Biopharm, SOCAR, Novo Nordisk, Uptton, Omeicos, and Boehringer Ingelheim. No other authors reported disclosures.
Figures

References
-
- World Health Organization. https://covid19.who.int. Accessed on Nov. 1, 2021.
-
- Liu Q, Wang RS, Qu GQ et al. Gross examination report of a COVID-19 death autopsy, Fa yi xue za zhi 36 (2020) 21–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
- 1OT2HL156812-01/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

