Heterogeneity in Lowe Syndrome: Mutations Affecting the Phosphatase Domain of OCRL1 Differ in Impact on Enzymatic Activity and Severity of Cellular Phenotypes
- PMID: 37189363
- PMCID: PMC10135975
- DOI: 10.3390/biom13040615
Heterogeneity in Lowe Syndrome: Mutations Affecting the Phosphatase Domain of OCRL1 Differ in Impact on Enzymatic Activity and Severity of Cellular Phenotypes
Abstract
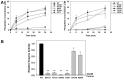
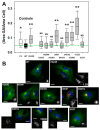
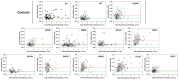
Lowe Syndrome (LS) is a condition due to mutations in the OCRL1 gene, characterized by congenital cataracts, intellectual disability, and kidney malfunction. Unfortunately, patients succumb to renal failure after adolescence. This study is centered in investigating the biochemical and phenotypic impact of patient's OCRL1 variants (OCRL1VAR). Specifically, we tested the hypothesis that some OCRL1VAR are stabilized in a non-functional conformation by focusing on missense mutations affecting the phosphatase domain, but not changing residues involved in binding/catalysis. The pathogenic and conformational characteristics of the selected variants were evaluated in silico and our results revealed some OCRL1VAR to be benign, while others are pathogenic. Then we proceeded to monitor the enzymatic activity and function in kidney cells of the different OCRL1VAR. Based on their enzymatic activity and presence/absence of phenotypes, the variants segregated into two categories that also correlated with the severity of the condition they induce. Overall, these two groups mapped to opposite sides of the phosphatase domain. In summary, our findings highlight that not every mutation affecting the catalytic domain impairs OCRL1's enzymatic activity. Importantly, data support the inactive-conformation hypothesis. Finally, our results contribute to establishing the molecular and structural basis for the observed heterogeneity in severity/symptomatology displayed by patients.
Keywords: Lowe syndrome; OCRL1; cellular phenotypes; phosphatase activity; rare genetic disease.
Conflict of interest statement
The authors declare no financial, personal or professional competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

