Anti-Citrullinated Protein Antibody Reactivity towards Neutrophil-Derived Antigens: Clonal Diversity and Inter-Individual Variation
- PMID: 37189377
- PMCID: PMC10135477
- DOI: 10.3390/biom13040630
Anti-Citrullinated Protein Antibody Reactivity towards Neutrophil-Derived Antigens: Clonal Diversity and Inter-Individual Variation
Abstract
Background: Why the adaptive immune system turns against citrullinated antigens in rheumatoid arthritis (RA) and whether anti-citrullinated protein antibodies (ACPAs) contribute to pathogenesis are questions that have triggered intense research, but still are not fully answered. Neutrophils may be crucial in this context, both as sources of citrullinated antigens and also as targets of ACPAs. To better understand how ACPAs and neutrophils contribute to RA, we studied the reactivity of a broad spectrum of RA patient-derived ACPA clones to activated or resting neutrophils, and we also compared neutrophil binding using polyclonal ACPAs from different patients.
Methods: Neutrophils were activated by Ca2+ ionophore, PMA, nigericin, zymosan or IL-8, and ACPA binding was studied using flow cytometry and confocal microscopy. The roles of PAD2 and PAD4 were studied using PAD-deficient mice or the PAD4 inhibitor BMS-P5.
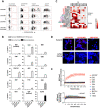
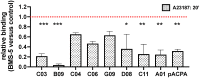
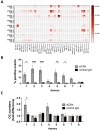
Results: ACPAs broadly targeted NET-like structures, but did not bind to intact cells or influence NETosis. We observed high clonal diversity in ACPA binding to neutrophil-derived antigens. PAD2 was dispensable, but most ACPA clones required PAD4 for neutrophil binding. Using ACPA preparations from different patients, we observed high patient-to-patient variability in targeting neutrophil-derived antigens and similarly in another cellular effect of ACPAs, the stimulation of osteoclast differentiation.
Conclusions: Neutrophils can be important sources of citrullinated antigens under conditions that lead to PAD4 activation, NETosis and the extrusion of intracellular material. A substantial clonal diversity in targeting neutrophils and a high variability among individuals in neutrophil binding and osteoclast stimulation suggest that ACPAs may influence RA-related symptoms with high patient-to-patient variability.
Keywords: ACPA; NETosis; citrullination; neutrophil; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Differential ACPA Binding to Nuclear Antigens Reveals a PAD-Independent Pathway and a Distinct Subset of Acetylation Cross-Reactive Autoantibodies in Rheumatoid Arthritis.Front Immunol. 2019 Jan 4;9:3033. doi: 10.3389/fimmu.2018.03033. eCollection 2018. Front Immunol. 2019. PMID: 30662440 Free PMC article.
-
Relative efficiencies of peptidylarginine deiminase 2 and 4 in generating target sites for anti-citrullinated protein antibodies in fibrinogen, alpha-enolase and histone H3.PLoS One. 2018 Aug 30;13(8):e0203214. doi: 10.1371/journal.pone.0203214. eCollection 2018. PLoS One. 2018. PMID: 30161253 Free PMC article.
-
Peptidylarginine deiminase 2 is required for tumor necrosis factor alpha-induced citrullination and arthritis, but not neutrophil extracellular trap formation.J Autoimmun. 2017 Jun;80:39-47. doi: 10.1016/j.jaut.2017.01.006. Epub 2017 Feb 7. J Autoimmun. 2017. PMID: 28188029 Free PMC article.
-
Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis.RMD Open. 2021 Mar;7(1):e001228. doi: 10.1136/rmdopen-2020-001228. RMD Open. 2021. PMID: 33771834 Free PMC article. Review.
-
Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis.Int J Mol Sci. 2020 Jun 4;21(11):4015. doi: 10.3390/ijms21114015. Int J Mol Sci. 2020. PMID: 32512739 Free PMC article. Review.
Cited by
-
The peculiar features, diversity and impact of citrulline-reactive autoantibodies.Nat Rev Rheumatol. 2024 Jul;20(7):399-416. doi: 10.1038/s41584-024-01124-6. Epub 2024 Jun 10. Nat Rev Rheumatol. 2024. PMID: 38858604 Review.
References
-
- Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J.S., Friday S., Li S., Patel R.M., Subramanian V., et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. - DOI - PMC - PubMed
-
- Harre U., Georgess D., Bang H., Bozec A., Axmann R., Ossipova E., Jakobsson P.-J., Baum W., Nimmerjahn F., Szarka E., et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012;122:1791–1802. doi: 10.1172/JCI60975. - DOI - PMC - PubMed
-
- Jurczak A., Delay L., Barbier J., Simon N., Krock E., Sandor K., Agalave N.M., Rudjito R., Wigerblad G., Rogóż K., et al. Antibody-induced pain-like behavior and bone erosion: Links to subclinical inflammation, osteoclast activity, and acid-sensing ion channel 3-dependent sensitization. Pain. 2021;163:1542–1559. doi: 10.1097/j.pain.0000000000002543. - DOI - PMC - PubMed
-
- Krishnamurthy A., Circiumaru A., Sun J., Kisten Y., Damberg P., Sakuraba K., Jarvoll P., Zhou T., Malmström V., Svensson C.I., et al. Combination of two monoclonal ACPAs induced tenosynovitis, pain and bone loss in mice in a Peptidyl Arginine Deiminase-4 dependent manner. Arthritis Rheumatol. 2022;75:164–170. doi: 10.1002/art.42320. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

