Endocardial Fibroelastosis as an Independent Predictor of Atrioventricular Valve Rupture in Maternal Autoimmune Antibody Exposed Fetus: A Systematic Review with Clinicopathologic Analysis
- PMID: 37189582
- PMCID: PMC10137427
- DOI: 10.3390/diagnostics13081481
Endocardial Fibroelastosis as an Independent Predictor of Atrioventricular Valve Rupture in Maternal Autoimmune Antibody Exposed Fetus: A Systematic Review with Clinicopathologic Analysis
Abstract
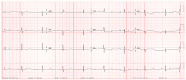
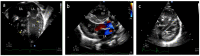
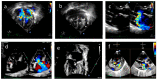
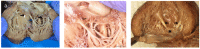
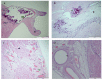
Background: Neonatal lupus (NL) is a clinical syndrome that develops in the fetus as a result of maternal autoimmune antibodies. Congenital complete heart block (CHB) is the most common manifestation, while extranodal cardiac manifestations of NL, such as endocardial fibroelastosis (EFE) and myocarditis, are rare but more serious. Less is known about this atrioventricular valve rupture due to valvulitis as a consequence of maternal autoantibodies. We have described a case of cardiac neonatal lupus with an antenatally detected CHB patient who developed mitral and tricuspid valve chordal rupture at 45 days of age. We compared the cardiac histopathology and the fetal cardiac echocardiographic findings of this case with another fetus that was aborted after being antenatally diagnosed with CHB but without valvar rupture. A narrative analysis after a systematic review of the literature regarding atrioventricular valve apparatus rupture due to autoimmune etiology along with maternal characteristics, presentation, treatment, and outcome have been discussed in this article.
Objectives: To describe published data on atrioventricular valve rupture in neonatal lupus, including clinical presentation, diagnostic evaluation, management, and outcomes.
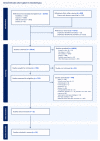
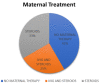
Methods: We conducted a PRISMA-compliant descriptive systematic examination of case reports that included accounts of lupus during pregnancy or in the newborn period that resulted in an atrioventricular valve rupture. We gathered information on the patient's demographics, the details of the valve rupture and other comorbidities, the maternal therapy, the clinical course, and the results. We also used a standardized method to evaluate the cases' quality. A total of 12 cases were investigated, with 11 cases drawn from 10 case reports or case series and 1 from our own experience.
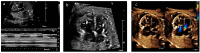
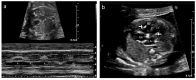
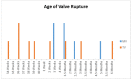
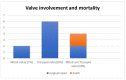
Results: Tricuspid valve rupture (50%) is more common than mitral valve rupture (17%). Unlike mitral valve rupture, which occurs postnatally, the timing of tricuspid valve rupture is perinatal. A total of 33% of the patients had concomitant complete heart block, while 75% of the patients had endocardial fibroelastosis on an antenatal ultrasound. Antenatal changes pertaining to endocardial fibroelastosis can be seen as early as 19 weeks of gestation. Patients with both valve ruptures generally have a poor prognosis, especially if they occur at close intervals.
Conclusion: Atrioventricular valve rupture in neonatal lupus is rare. A majority of patients with valve rupture had antenatally detected endocardial fibroelastosis in the valvar apparatus. Appropriate and expedited surgical repair of ruptured atrioventricular valves is feasible and has a low mortality risk. Rupture of both atrioventricular valves occurring at close intervals carries a high mortality risk.
Keywords: anti La/SSB antibodies; anti-Ro/SSA antibodies; complete heart block; endocardial fibroelastosis; maternal autoantibodies; mitral valve rupture; neonatal lupus; tricuspid valve rupture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Izmirly P.M., Saxena A., Kim M.Y., Wang D., Sahl S.K., Llanos C., Friedman D., Buyon J.P. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. - DOI - PMC - PubMed
-
- Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Qureshi R., Mattis P., Lisy K. JBI Critical appraisal checklist for case reports. Joanna Briggs Inst. Rev. Man. Joanna Briggs Inst. 2017:1–7.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

