Acellular Biomaterials Associated with Autologous Bone Marrow-Derived Mononuclear Stem Cells Improve Wound Healing through Paracrine Effects
- PMID: 37189621
- PMCID: PMC10136340
- DOI: 10.3390/biomedicines11041003
Acellular Biomaterials Associated with Autologous Bone Marrow-Derived Mononuclear Stem Cells Improve Wound Healing through Paracrine Effects
Abstract
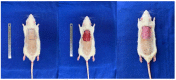
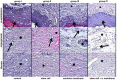
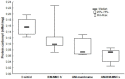
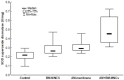
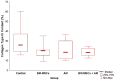
Wound healing is a complex process of repair that involves the interaction between different cell types and involves coordinated interactions between intracellular and extracellular signaling. Bone Marrow Mesenchymal Stem Cells (BMSCs) based and acellular amniotic membrane (AM) therapeutic strategies with the potential for treatment and regeneration of tissue. We aimed to evaluate the involvement of paracrine effects in tissue repair after the flap skin lesion rat model. In the full-thickness flap skin experiment of forty Wistar rats: A total of 40 male Wistar rats were randomized into four groups: group I: control (C; n = 10), with full-thickness lesions on the back, without (BMSCs) or AM (n = 10); group II: injected (BMSCs; n = 10); group III: covered by AM; group IV-injected (AM + BMSCs; n = 10). Cytokine levels, IL-1, and IL-10 assay kits, superoxide dismutase (SOD), glutathione reductase (GRs) and carbonyl activity levels were measured by ELISA 28th day, and TGF-β was evaluated by immunohistochemical, the expression collagen expression was evaluated by Picrosirius staining. Our results showed that the IL-1 interleukin was higher in the control group, and the IL-10 presented a higher mean when compared to the control group. The groups with BMSCs and AM showed the lowest expression levels of TGF-β. SOD, GRs, and carbonyl activity analysis showed a predominance in groups that received treatment from 80%. The collagen fiber type I was predominant in all groups; however, the AM + BMSCs group obtained a higher average when compared to the control group. Our findings suggest that the AM+ BMSCs promote skin wound healing, probably owing to their paracrine effect attributed to the promotion of new collagen for tissue repair.
Keywords: antioxidant system; experimental animal models; human acellular amniotic membrane; paracrine; stem cells; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

