Streptomyces Bioactive Metabolites Prevent Liver Cancer through Apoptosis, Inhibiting Oxidative Stress and Inflammatory Markers in Diethylnitrosamine-Induced Hepatocellular Carcinoma
- PMID: 37189672
- PMCID: PMC10135527
- DOI: 10.3390/biomedicines11041054
Streptomyces Bioactive Metabolites Prevent Liver Cancer through Apoptosis, Inhibiting Oxidative Stress and Inflammatory Markers in Diethylnitrosamine-Induced Hepatocellular Carcinoma
Abstract
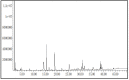
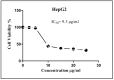
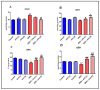
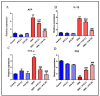
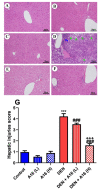
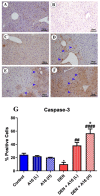
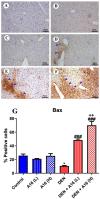
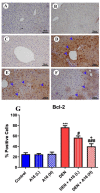
A safe and effective treatment for liver cancer is still elusive despite all attempts. Biomolecules produced from natural products and their derivatives are potential sources of new anticancer medications. This study aimed to investigate the anticancer potential of a Streptomyces sp. bacterial extract against diethylnitrosamine (DEN)-induced liver cancer in Swiss albino mice and explore the underlying cellular and molecular mechanisms. The ethyl acetate extract of a Streptomyces sp. was screened for its potential anticancer activities against HepG-2 using the MTT assay, and the IC50 was also determined. Gas chromatography-mass spectrometric analysis was used to identify the chemical constituents of the Streptomyces extract. Mice were administered DEN at the age of 2 weeks, and from week 32 until week 36 (4 weeks), they received two doses of Streptomyces extract (25 and 50 mg/kg body weight) orally daily. The Streptomyces extract contains 29 different compounds, according to the GC-MS analysis. The rate of HepG-2 growth was dramatically reduced by the Streptomyces extract. In the mice model. Streptomyces extract considerably lessened the negative effects of DEN on liver functions at both doses. Alpha-fetoprotein (AFP) levels were significantly (p < 0.001) decreased, and P53 mRNA expression was increased, both of which were signs that Streptomyces extract was suppressing carcinogenesis. This anticancer effect was also supported by histological analysis. Streptomyces extract therapy additionally stopped DEN-induced alterations in hepatic oxidative stress and enhanced antioxidant activity. Additionally, Streptomyces extract reduced DEN-induced inflammation, as shown by the decline in interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) levels. Additionally, the Streptomyces extract administration dramatically boosted Bax and caspase-3 levels while decreasing Bcl-2 expressions in the liver according to the Immunohistochemistry examination. In summary, Streptomyces extract is reported here as a potent chemopreventive agent against hepatocellular carcinoma through multiple mechanisms, including inhibiting oxidative stress, cell apoptosis, and inflammation.
Keywords: Streptomyces; anticancer activity; apoptosis; hepatocellular carcinoma; inflammation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Nimbolide inhibits tumor growth by restoring hepatic tight junction protein expression and reduced inflammation in an experimental hepatocarcinogenesis.World J Gastroenterol. 2020 Dec 7;26(45):7131-7152. doi: 10.3748/wjg.v26.i45.7131. World J Gastroenterol. 2020. PMID: 33362373 Free PMC article.
-
Anti-carcinogenic effects and mechanisms of actions of Citrus limon fruit peel hydroethanolic extract and limonene in diethylnitrosmine/2-acetylaminofluorene-induced hepatocellular carcinoma in Wistar rats.Am J Cancer Res. 2024 Nov 15;14(11):5193-5215. doi: 10.62347/FOYI6658. eCollection 2024. Am J Cancer Res. 2024. PMID: 39659918 Free PMC article.
-
Nelumbo nucifera leaf extract treatment attenuated preneoplastic lesions and oxidative stress in the livers of diethylnitrosamine-treated rats.Environ Toxicol. 2017 Nov;32(11):2327-2340. doi: 10.1002/tox.22434. Epub 2017 Aug 14. Environ Toxicol. 2017. PMID: 28804948
-
Annona senegalensis extract demonstrates anticancer properties in N-diethylnitrosamine-induced hepatocellular carcinoma in male Wistar rats.Biomed Pharmacother. 2020 Nov;131:110786. doi: 10.1016/j.biopha.2020.110786. Epub 2020 Sep 30. Biomed Pharmacother. 2020. PMID: 33152944
-
Potential anticancer effects of cyclo(-Pro-Tyr) against N-diethyl nitrosamine induced hepatocellular carcinoma in mouse through PI3K/AKT signaling.Environ Toxicol. 2022 Feb;37(2):256-269. doi: 10.1002/tox.23395. Epub 2021 Nov 2. Environ Toxicol. 2022. PMID: 34726822
Cited by
-
2-Amino-3-Chlorobenzoic Acid from Streptomyces coelicolor: A Cancer Antagonist Targeting PI3K/AKT Markers via miRNA Modulation.Pharmaceuticals (Basel). 2025 Apr 24;18(5):620. doi: 10.3390/ph18050620. Pharmaceuticals (Basel). 2025. PMID: 40430441 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

