Formulation and Optimization of Repaglinide Nanoparticles Using Microfluidics for Enhanced Bioavailability and Management of Diabetes
- PMID: 37189682
- PMCID: PMC10135525
- DOI: 10.3390/biomedicines11041064
Formulation and Optimization of Repaglinide Nanoparticles Using Microfluidics for Enhanced Bioavailability and Management of Diabetes
Erratum in
-
Correction: Ahmad et al. Formulation and Optimization of Repaglinide Nanoparticles Using Microfluidics for Enhanced Bioavailability and Management of Diabetes. Biomedicines 2023, 11, 1064.Biomedicines. 2025 Nov 17;13(11):2795. doi: 10.3390/biomedicines13112795. Biomedicines. 2025. PMID: 41301936 Free PMC article.
Abstract
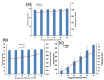
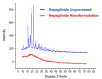
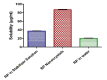
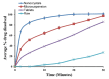
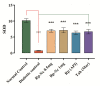
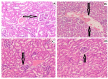
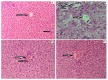
The technologies for fabrication of nanocrystals have an immense potential to improve solubility of a variety of the poor water-soluble drugs with subsequent enhanced bioavailability. Repaglinide (Rp) is an antihyperglycemic drug having low bioavailability due to its extensive first-pass metabolism. Microfluidics is a cutting-edge technique that provides a new approach for producing nanoparticles (NPs) with controlled properties for a variety of applications. The current study's goal was to engineer repaglinide smart nanoparticles (Rp-Nc) utilizing microfluidic technology (Dolomite Y shape), and then to perform in-vitro, in-vivo, and toxicity evaluations of them. This method effectively generated nanocrystals with average particle sizes of 71.31 ± 11 nm and a polydispersity index (PDI) of 0.072 ± 12. The fabricated Rp's crystallinity was verified by Differential scanning calorimetry (DSC) and Powder X-ray diffraction (PXRD). In comparison to the raw and commercially available tablets, the fabricated Rp's nanoparticles resulted in a higher saturation solubility and dissolving rate (p < 0.05). Rp nanocrystals had a considerably lower (p < 0.05) IC50 value than that of the raw drug and commercial tablets. Moreover, Rp nanocrystals at the 0.5 and 1 mg/kg demonstrated a significant decrease in blood glucose level (mg/dL, p < 0.001, n = 8) compared to its counterparts. Rp nanocrystals at the 0.5 mg/kg demonstrated a significant decrease (p < 0.001, n = 8) in blood glucose compared to its counterparts at a dose of 1 mg/kg. The selected animal model's histological analyses and the effect of Rp nanocrystals on several internal organs were determined to be equivalent to those of the control animal group. The findings of the present study indicated that nanocrystals of Rp with improved anti-diabetic properties and safety profiles can be successfully produced using controlled microfluidic technology, an innovative drug delivery system (DDS) approach.
Keywords: bioavailability; in-vitro; in-vivo study; microfluidic technology; rats; repaglinide (Rp).
Conflict of interest statement
The authors of this article have no conflict of interest.
Figures




References
-
- Patle D., Vyas M., Khatik G.L. A review on natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021;17:186–197. - PubMed
-
- Patterson C.C., Karuranga S., Salpea P., Saeedi P., Dahlquist G., Soltesz G., Ogle G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019;157:107842. doi: 10.1016/j.diabres.2019.107842. - DOI - PubMed
LinkOut - more resources
Full Text Sources

