Temozolomide Resistance in Glioblastoma by NRF2: Protecting the Evil
- PMID: 37189700
- PMCID: PMC10136186
- DOI: 10.3390/biomedicines11041081
Temozolomide Resistance in Glioblastoma by NRF2: Protecting the Evil
Abstract
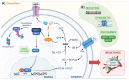
The transcription factor NRF2 is constitutively active in glioblastoma, a highly aggressive brain tumor subtype with poor prognosis. Temozolomide (TMZ) is the primary chemotherapeutic agent for this type of tumor treatment, but resistance to this drug is often observed. This review highlights the research that is demonstrating how NRF2 hyperactivation creates an environment that favors the survival of malignant cells and protects against oxidative stress and TMZ. Mechanistically, NRF2 increases drug detoxification, autophagy, DNA repair, and decreases drug accumulation and apoptotic signaling. Our review also presents potential strategies for targeting NRF2 as an adjuvant therapy to overcome TMZ chemoresistance in glioblastoma. Specific molecular pathways, including MAPKs, GSK3β, βTRCP, PI3K, AKT, and GBP, that modulate NRF2 expression leading to TMZ resistance are discussed, along with the importance of identifying NRF2 modulators to reverse TMZ resistance and develop new therapeutic targets. Despite the significant progress in understanding the role of NRF2 in GBM, there are still unanswered questions regarding its regulation and downstream effects. Future research should focus on elucidating the precise mechanisms by which NRF2 mediates resistance to TMZ, and identifying potential novel targets for therapeutic intervention.
Keywords: NRF2; glioblastoma; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

