Uncoupling Lipid Synthesis from Adipocyte Development
- PMID: 37189751
- PMCID: PMC10135928
- DOI: 10.3390/biomedicines11041132
Uncoupling Lipid Synthesis from Adipocyte Development
Abstract
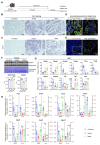
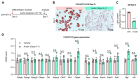
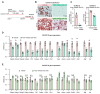
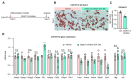
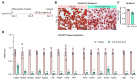
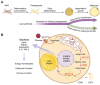
Obesity results from the expansion of adipose tissue, a versatile tissue regulating energy homeostasis, adipokine secretion, thermogenesis, and inflammation. The primary function of adipocytes is thought to be lipid storage through lipid synthesis, which is presumably intertwined with adipogenesis. However, during prolonged fasting, adipocytes are depleted of lipid droplets yet retain endocrine function and an instant response to nutrients. This observation led us to question whether lipid synthesis and storage can be uncoupled from adipogenesis and adipocyte function. By inhibiting key enzymes in the lipid synthesis pathway during adipocyte development, we demonstrated that a basal level of lipid synthesis is essential for adipogenesis initiation but not for maturation and maintenance of adipocyte identity. Furthermore, inducing dedifferentiation of mature adipocytes abrogated adipocyte identity but not lipid storage. These findings suggest that lipid synthesis and storage are not the defining features of adipocytes and raise the possibility of uncoupling lipid synthesis from adipocyte development to achieve smaller and healthier adipocytes for the treatment of obesity and related disorders.
Keywords: ACC; DGAT; FASN; adipocyte; adipogenesis; fatty acid; lipogenesis; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Murray C.J.L., Aravkin A.Y., Zheng P., Abbafati C., Abbas K.M., Abbasi-Kangevari M., Abd-Allah F., Abdelalim A., Abdollahi M., Abdollahpour I., et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. - DOI - PMC - PubMed
-
- Bhatti G.K., Bhadada S.K., Vijayvergiya R., Mastana S.S., Bhatti J.S. Metabolic syndrome and risk of major coronary events among the urban diabetic patients: North Indian Diabetes and Cardiovascular Disease Study—NIDCVD-2. J. Diabetes Complicat. 2016;30:72–78. doi: 10.1016/j.jdiacomp.2015.07.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

