Controversial Properties of Amyloidogenic Proteins and Peptides: New Data in the COVID Era
- PMID: 37189833
- PMCID: PMC10136278
- DOI: 10.3390/biomedicines11041215
Controversial Properties of Amyloidogenic Proteins and Peptides: New Data in the COVID Era
Abstract
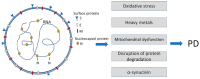
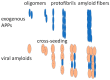
For a long time, studies of amyloidogenic proteins and peptides (amyloidogenic PPs) have been focused basically on their harmful properties and association with diseases. A vast amount of research has investigated the structure of pathogenic amyloids forming fibrous deposits within or around cells and the mechanisms of their detrimental actions. Much less has been known about the physiologic functions and beneficial properties of amyloidogenic PPs. At the same time, amyloidogenic PPs have various useful properties. For example, they may render neurons resistant to viral infection and propagation and stimulate autophagy. We discuss here some of amyloidogenic PPs' detrimental and beneficial properties using as examples beta-amyloid (β-amyloid), implicated in the pathogenesis of Alzheimer's disease (AD), and α-synuclein-one of the hallmarks of Parkinson's disease (PD). Recently amyloidogenic PPs' antiviral and antimicrobial properties have attracted attention because of the COVID-19 pandemic and the growing threat of other viral and bacterial-induced diseases. Importantly, several COVID-19 viral proteins, e.g., spike, nucleocapsid, and envelope proteins, may become amyloidogenic after infection and combine their harmful action with the effect of endogenous APPs. A central area of current investigations is the study of the structural properties of amyloidogenic PPs, defining their beneficial and harmful properties, and identifying triggers that transform physiologically important amyloidogenic PPs into vicious substances. These directions are of paramount importance during the current SARS-CoV-2 global health crisis.
Keywords: Alzheimer’s disease; COVID-19; Parkinson’s disease; SARS-CoV-2; amyloidogenic peptides; amyloidogenic proteins; amyloidosis; α-synuclein; β-amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

