Experiences of Caregivers and At-Risk Children Enrolled in a Prospective Pregnancy-Birth Cohort Study into the Causes of Type 1 Diabetes: The ENDIA Study
- PMID: 37189886
- PMCID: PMC10137087
- DOI: 10.3390/children10040637
Experiences of Caregivers and At-Risk Children Enrolled in a Prospective Pregnancy-Birth Cohort Study into the Causes of Type 1 Diabetes: The ENDIA Study
Abstract
Background: We sought research experiences of caregivers and their children were enrolled in the Environmental Determinants of Islet Autoimmunity (ENDIA) study.
Methods: ENDIA is a pregnancy-birth cohort investigating early-life causes of type 1 diabetes (T1D). Surveys were sent to 1090 families between June 2021 and March 2022 with a median participation of >5 years. Caregivers completed a 12-item survey. Children ≥ 3 years completed a four-item survey.
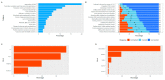
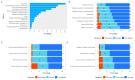
Results: The surveys were completed by 550/1090 families (50.5%) and 324/847 children (38.3%). The research experience was rated as either "excellent" or "good" by 95% of caregivers, and 81% of children were either "ok", "happy" or "very happy". The caregivers were motivated by contributing to research and monitoring their children for T1D. Relationships with the research staff influenced the experience. The children most liked virtual reality headsets, toys, and "helping". Blood tests were least liked by the children and were the foremost reason that 23.4% of the caregivers considered withdrawing. The children valued gifts more than their caregivers. Only 5.9% of responses indicated dissatisfaction with some aspects of the protocol. The self-collection of samples in regional areas, or during the COVID-19 pandemic restrictions, were accepted.
Conclusions: This evaluation identified modifiable protocol elements and was conducted to further improve satisfaction. What was important to the children was distinct from their caregivers.
Keywords: cohort study; consumer and community involvement; consumer engagement; evaluation; type 1 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

