Inflammation as a Regulator of the Airway Surface Liquid pH in Cystic Fibrosis
- PMID: 37190013
- PMCID: PMC10137218
- DOI: 10.3390/cells12081104
Inflammation as a Regulator of the Airway Surface Liquid pH in Cystic Fibrosis
Abstract
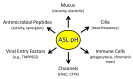
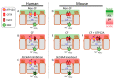
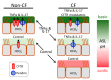
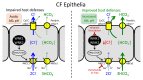
The airway surface liquid (ASL) is a thin sheet of fluid that covers the luminal aspect of the airway epithelium. The ASL is a site of several first-line host defenses, and its composition is a key factor that determines respiratory fitness. Specifically, the acid-base balance of ASL has a major influence on the vital respiratory defense processes of mucociliary clearance and antimicrobial peptide activity against inhaled pathogens. In the inherited disorder cystic fibrosis (CF), loss of cystic fibrosis transmembrane conductance regulator (CFTR) anion channel function reduces HCO3- secretion, lowers the pH of ASL (pHASL), and impairs host defenses. These abnormalities initiate a pathologic process whose hallmarks are chronic infection, inflammation, mucus obstruction, and bronchiectasis. Inflammation is particularly relevant as it develops early in CF and persists despite highly effective CFTR modulator therapy. Recent studies show that inflammation may alter HCO3- and H+ secretion across the airway epithelia and thus regulate pHASL. Moreover, inflammation may enhance the restoration of CFTR channel function in CF epithelia exposed to clinically approved modulators. This review focuses on the complex relationships between acid-base secretion, airway inflammation, pHASL regulation, and therapeutic responses to CFTR modulators. These factors have important implications for defining optimal ways of tackling CF airway inflammation in the post-modulator era.
Keywords: airway epithelium; airway surface liquid; cystic fibrosis; host defense; inflammation; pH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators.J Clin Invest. 2021 Aug 16;131(16):e150398. doi: 10.1172/JCI150398. J Clin Invest. 2021. PMID: 34166230 Free PMC article.
-
Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):16083-8. doi: 10.1073/pnas.2634339100. Epub 2003 Dec 10. Proc Natl Acad Sci U S A. 2003. PMID: 14668433 Free PMC article.
-
Relationships among CFTR expression, HCO3- secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies.Proc Natl Acad Sci U S A. 2016 May 10;113(19):5382-7. doi: 10.1073/pnas.1604905113. Epub 2016 Apr 25. Proc Natl Acad Sci U S A. 2016. PMID: 27114540 Free PMC article.
-
Modulation of Ion Transport to Restore Airway Hydration in Cystic Fibrosis.Genes (Basel). 2021 Mar 22;12(3):453. doi: 10.3390/genes12030453. Genes (Basel). 2021. PMID: 33810137 Free PMC article. Review.
-
Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets.Thorax. 2016 Mar;71(3):284-7. doi: 10.1136/thoraxjnl-2015-207588. Epub 2015 Dec 30. Thorax. 2016. PMID: 26719229 Review.
Cited by
-
Putting bicarbonate on the spot: pharmacological insights for CFTR correction in the airway epithelium.Front Pharmacol. 2023 Dec 11;14:1293578. doi: 10.3389/fphar.2023.1293578. eCollection 2023. Front Pharmacol. 2023. PMID: 38149052 Free PMC article.
-
Epithelial responses to CFTR modulators are improved by inflammatory cytokines and impaired by antiinflammatory drugs.JCI Insight. 2024 Jun 18;9(14):e181836. doi: 10.1172/jci.insight.181836. JCI Insight. 2024. PMID: 38888974 Free PMC article.
-
Sex differences in airway disease: estrogen and airway surface liquid dynamics.Biol Sex Differ. 2024 Jul 18;15(1):56. doi: 10.1186/s13293-024-00633-z. Biol Sex Differ. 2024. PMID: 39026347 Free PMC article. Review.
-
IL-13 decreases susceptibility to airway epithelial SARS-CoV-2 infection but increases disease severity in vivo.bioRxiv [Preprint]. 2024 Jul 4:2024.07.03.601941. doi: 10.1101/2024.07.03.601941. bioRxiv. 2024. PMID: 39005257 Free PMC article. Preprint.
-
Dynamic measurement of airway surface liquid volume with an ex vivo trachea-chip.Lab Chip. 2024 Jun 11;24(12):3093-3100. doi: 10.1039/d4lc00134f. Lab Chip. 2024. PMID: 38779981 Free PMC article.
References
-
- Widdicombe J.H. Airway Epithelium. Morgan & Claypool; San Rafael, CA, USA: 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

