Induction of miR-665-3p Impairs the Differentiation of Myogenic Progenitor Cells by Regulating the TWF1-YAP1 Axis
- PMID: 37190023
- PMCID: PMC10136822
- DOI: 10.3390/cells12081114
Induction of miR-665-3p Impairs the Differentiation of Myogenic Progenitor Cells by Regulating the TWF1-YAP1 Axis
Abstract
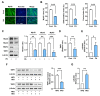
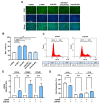
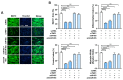
Actin dynamics are known to orchestrate various myogenic processes in progenitor cells. Twinfilin-1 (TWF1) is an actin-depolymerizing factor that plays a crucial role in the differentiation of myogenic progenitor cells. However, little is known about the mechanisms underlying the epigenetic regulation of TWF1 expression and impaired myogenic differentiation in the background of muscle wasting. This study investigated how miR-665-3p affects TWF1 expression, actin filaments' modulation, proliferation, and myogenic differentiation in progenitor cells. Palmitic acid, the most prevalent saturated fatty acid (SFA) in food, suppressed TWF1 expression and inhibited the myogenic differentiation of C2C12 cells while increasing the level of miR-665-3p expression. Interestingly, miR-665-3p inhibited TWF1 expression by targeting TWF1 3'UTR directly. In addition, miR-665-3p accumulated filamentous actin (F-actin) and enhanced the nuclear translocation of Yes-associated protein 1 (YAP1), consequently promoting cell cycle progression and proliferation. Furthermore, miR-665-3p suppressed the expressions of myogenic factors, i.e., MyoD, MyoG, and MyHC, and consequently impaired myoblast differentiation. In conclusion, this study suggests that SFA-inducible miR-665-3p suppresses TWF1 expression epigenetically and inhibits myogenic differentiation by facilitating myoblast proliferation via the F-actin/YAP1 axis.
Keywords: F-actin; YAP1; actin dynamics; differentiation; miR-665-3p; myogenesis; obesity; proliferation; twinfilin-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mir-302a/TWF1 Axis Impairs the Myogenic Differentiation of Progenitor Cells through F-Actin-Mediated YAP1 Activation.Int J Mol Sci. 2023 Mar 28;24(7):6341. doi: 10.3390/ijms24076341. Int J Mol Sci. 2023. PMID: 37047312 Free PMC article.
-
Saturated fatty acid-inducible miR-103-3p impairs the myogenic differentiation of progenitor cells by enhancing cell proliferation through Twinfilin-1/F-actin/YAP1 axis.Korean J Physiol Pharmacol. 2023 May 1;27(3):277-287. doi: 10.4196/kjpp.2023.27.3.277. Korean J Physiol Pharmacol. 2023. PMID: 37078301 Free PMC article.
-
MiR-320-3p Regulates the Proliferation and Differentiation of Myogenic Progenitor Cells by Modulating Actin Remodeling.Int J Mol Sci. 2022 Jan 12;23(2):801. doi: 10.3390/ijms23020801. Int J Mol Sci. 2022. PMID: 35054986 Free PMC article.
-
Twinfilin-1 is an essential regulator of myogenic differentiation through the modulation of YAP in C2C12 myoblasts.Biochem Biophys Res Commun. 2022 Apr 9;599:17-23. doi: 10.1016/j.bbrc.2022.02.021. Epub 2022 Feb 8. Biochem Biophys Res Commun. 2022. PMID: 35168059
-
Myogenic Progenitor Cell Differentiation Is Dependent on Modulation of Mitochondrial Biogenesis through Autophagy.2016 Jun 25. In: Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]. Tokyo: Springer; 2016. Chapter 15. 2016 Jun 25. In: Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]. Tokyo: Springer; 2016. Chapter 15. PMID: 29787148 Free Books & Documents. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

