Preclinical Studies with Glioblastoma Brain Organoid Co-Cultures Show Efficient 5-ALA Photodynamic Therapy
- PMID: 37190034
- PMCID: PMC10137158
- DOI: 10.3390/cells12081125
Preclinical Studies with Glioblastoma Brain Organoid Co-Cultures Show Efficient 5-ALA Photodynamic Therapy
Abstract
Background: The high recurrence of glioblastoma (GB) that occurs adjacent to the resection cavity within two years of diagnosis urges an improvement of therapies oriented to GB local control. Photodynamic therapy (PDT) has been proposed to cleanse infiltrating tumor cells from parenchyma to ameliorate short long-term progression-free survival. We examined 5-aminolevulinic acid (5-ALA)-mediated PDT effects as therapeutical treatment and determined optimal conditions for PDT efficacy without causing phototoxic injury to the normal brain tissue.
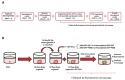
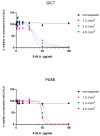
Methods: We used a platform of Glioma Initiation Cells (GICs) infiltrating cerebral organoids with two different glioblastoma cells, GIC7 and PG88. We measured GICs-5-ALA uptake and PDT/5-ALA activity in dose-response curves and the efficacy of the treatment by measuring proliferative activity and apoptosis.
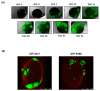
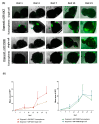
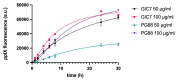
Results: 5-ALA (50 and 100 µg/mL) was applied, and the release of protoporphyrin IX (PpIX) fluorescence measures demonstrated that the emission of PpIX increases progressively until its stabilization at 24 h. Moreover, decreased proliferation and increased apoptosis corroborated the effect of 5-ALA/PDT on cancer cells without altering normal cells.
Conclusions: We provide evidence about the effectiveness of PDT to treat high proliferative GB cells in a complex in vitro system, which combines normal and cancer cells and is a useful tool to standardize new strategic therapies.
Keywords: 3D tumor models; 5-ALA; drug screening; glioblastoma; neurological cancers; organoids; personalized medicine; photodynamic therapy; spheroids.
Conflict of interest statement
Marc Centellas and Marta Muñoz-Tudurí are employees of Laboratorios Gebro Pharma S.A.; the rest of the authors declare no conflict of interest.
Figures





References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

