Distinct Responses to IL4 in Macrophages Mediated by JNK
- PMID: 37190036
- PMCID: PMC10136765
- DOI: 10.3390/cells12081127
Distinct Responses to IL4 in Macrophages Mediated by JNK
Abstract
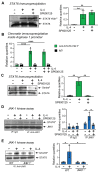
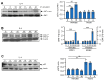
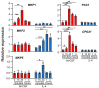
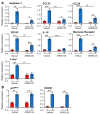
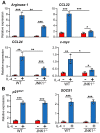
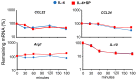
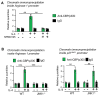
IL(Interleukin)-4 is the main macrophage M2-type activator and induces an anti-inflammatory phenotype called alternative activation. The IL-4 signaling pathway involves the activation of STAT (Signal Transducer and Activator of Transcription)-6 and members of the MAPK (Mitogen-activated protein kinase) family. In primary-bone-marrow-derived macrophages, we observed a strong activation of JNK (Jun N-terminal kinase)-1 at early time points of IL-4 stimulation. Using selective inhibitors and a knockout model, we explored the contribution of JNK-1 activation to macrophages' response to IL-4. Our findings indicate that JNK-1 regulates the IL-4-mediated expression of genes typically involved in alternative activation, such as Arginase 1 or Mannose receptor, but not others, such as SOCS (suppressor of cytokine signaling) 1 or p21Waf-1 (cyclin dependent kinase inhibitor 1A). Interestingly, we have observed that after macrophages are stimulated with IL-4, JNK-1 has the capacity to phosphorylate STAT-6 on serine but not on tyrosine. Chromatin immunoprecipitation assays revealed that functional JNK-1 is required for the recruitment of co-activators such as CBP (CREB-binding protein)/p300 on the promoter of Arginase 1 but not on p21Waf-1. Taken together, these data demonstrate the critical role of STAT-6 serine phosphorylation by JNK-1 in distinct macrophage responses to IL-4.
Keywords: chemokines; cytokines; inflammation; kinases/phosphatases; monocytes/macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

