Astroglial Connexin 43 Regulates Synaptic Vesicle Release at Hippocampal Synapses
- PMID: 37190042
- PMCID: PMC10136730
- DOI: 10.3390/cells12081133
Astroglial Connexin 43 Regulates Synaptic Vesicle Release at Hippocampal Synapses
Abstract
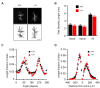
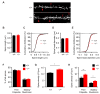
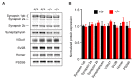
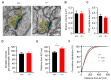
Connexin 43, an astroglial gap junction protein, is enriched in perisynaptic astroglial processes and plays major roles in synaptic transmission. We have previously found that astroglial Cx43 controls synaptic glutamate levels and allows for activity-dependent glutamine release to sustain physiological synaptic transmissions and cognitiogns. However, whether Cx43 is important for the release of synaptic vesicles, which is a critical component of synaptic efficacy, remains unanswered. Here, using transgenic mice with a glial conditional knockout of Cx43 (Cx43-/-), we investigate whether and how astrocytes regulate the release of synaptic vesicles from hippocampal synapses. We report that CA1 pyramidal neurons and their synapses develop normally in the absence of astroglial Cx43. However, a significant impairment in synaptic vesicle distribution and release dynamics were observed. In particular, the FM1-43 assays performed using two-photon live imaging and combined with multi-electrode array stimulation in acute hippocampal slices, revealed a slower rate of synaptic vesicle release in Cx43-/- mice. Furthermore, paired-pulse recordings showed that synaptic vesicle release probability was also reduced and is dependent on glutamine supply via Cx43 hemichannel (HC). Taken together, we have uncovered a role for Cx43 in regulating presynaptic functions by controlling the rate and probability of synaptic vesicle release. Our findings further highlight the significance of astroglial Cx43 in synaptic transmission and efficacy.
Keywords: astrocytes; connexin 43; hippocampus; release probability; synaptic transmission; synaptic vesicle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Danbolt N.C. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

