Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium
- PMID: 37190073
- PMCID: PMC10137269
- DOI: 10.3390/cells12081164
Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium
Abstract
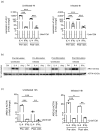
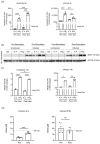
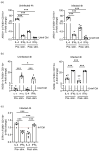
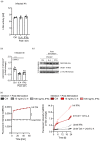
Priming of macrophages with interferon-gamma (IFNγ) or interleukin-4 (IL-4) leads to polarisation into pro-inflammatory or anti-inflammatory subtypes, which produce key enzymes such as inducible nitric oxide synthase (iNOS) and arginase 1 (ARG1), respectively, and in this way determine host responses to infection. Importantly, L-arginine is the substrate for both enzymes. ARG1 upregulation is associated with increased pathogen load in different infection models. However, while differentiation of macrophages with IL-4 impairs host resistance to the intracellular bacterium Salmonella enterica serovar Typhimurium (S.tm), little is known on the effects of IL-4 on unpolarised macrophages during infection. Therefore, bone-marrow-derived macrophages (BMDM) from C57BL/6N, Tie2Cre+/-ARG1fl/fl (KO), Tie2Cre-/-ARG1fl/fl (WT) mice were infected with S.tm in the undifferentiated state and then stimulated with IL-4 or IFNγ. In addition, BMDM of C57BL/6N mice were first polarised upon stimulation with IL-4 or IFNγ and then infected with S.tm. Interestingly, in contrast to polarisation of BMDM with IL-4 prior to infection, treatment of non-polarised S.tm-infected BMDM with IL-4 resulted in improved infection control whereas stimulation with IFNγ led to an increase in intracellular bacterial numbers compared to unstimulated controls. This effect of IL-4 was paralleled by decreased ARG1 levels and increased iNOS expression. Furthermore, the L-arginine pathway metabolites ornithine and polyamines were enriched in unpolarised cells infected with S.tm and stimulated with IL-4. Depletion of L-arginine reversed the protective effect of IL-4 toward infection control. Our data show that stimulation of S.tm-infected macrophages with IL-4 reduced bacterial multiplication via metabolic re-programming of L-arginine-dependent pathways.
Keywords: Salmonella enterica serovar Typhimurium; arginase 1; inducible nitric oxide synthase; interferon-gamma; interleukin-4; intracellular bacteria; macrophages; ornithine; polyamines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bosurgi L., Cao Y.G., Cabeza-Cabrerizo M., Tucci A., Hughes L.D., Kong Y., Weinstein J.S., Licona-Limon P., Schmid E.T., Pelorosso F., et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356:1072–1076. doi: 10.1126/science.aai8132. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

