Direct Reprogramming of Resident Non-Myocyte Cells and Its Potential for In Vivo Cardiac Regeneration
- PMID: 37190075
- PMCID: PMC10136631
- DOI: 10.3390/cells12081166
Direct Reprogramming of Resident Non-Myocyte Cells and Its Potential for In Vivo Cardiac Regeneration
Abstract
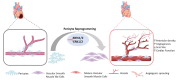
Cardiac diseases are the foremost cause of morbidity and mortality worldwide. The heart has limited regenerative potential; therefore, lost cardiac tissue cannot be replenished after cardiac injury. Conventional therapies are unable to restore functional cardiac tissue. In recent decades, much attention has been paid to regenerative medicine to overcome this issue. Direct reprogramming is a promising therapeutic approach in regenerative cardiac medicine that has the potential to provide in situ cardiac regeneration. It consists of direct cell fate conversion of one cell type into another, avoiding transition through an intermediary pluripotent state. In injured cardiac tissue, this strategy directs transdifferentiation of resident non-myocyte cells (NMCs) into mature functional cardiac cells that help to restore the native tissue. Over the years, developments in reprogramming methods have suggested that regulation of several intrinsic factors in NMCs can help to achieve in situ direct cardiac reprogramming. Among NMCs, endogenous cardiac fibroblasts have been studied for their potential to be directly reprogrammed into both induced cardiomyocytes and induced cardiac progenitor cells, while pericytes can transdifferentiate towards endothelial cells and smooth muscle cells. This strategy has been indicated to improve heart function and reduce fibrosis after cardiac injury in preclinical models. This review summarizes the recent updates and progress in direct cardiac reprogramming of resident NMCs for in situ cardiac regeneration.
Keywords: cardiac fibroblasts; cardiac injury; cardiac regeneration; cardiac repair; direct reprogramming; induced cardiac progenitor cells; induced cardiomyocytes; non-myocytic cells; pericytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vitale E., Rosso R., Lo Iacono M., Cristallini C., Giachino C., Rastaldo R. Apelin-13 Increases Functional Connexin-43 through Autophagy Inhibition via AKT/mTOR Pathway in the Non-Myocytic Cell Population of the Heart. Int. J. Mol. Sci. 2022;23:13073. doi: 10.3390/ijms232113073. - DOI - PMC - PubMed

