A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages
- PMID: 37190098
- PMCID: PMC10137183
- DOI: 10.3390/cells12081189
A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages
Abstract
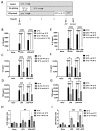
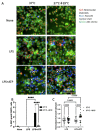
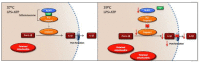
Inflammation and mitochondrial-dependent oxidative stress are interrelated processes implicated in multiple neuroinflammatory disorders, including Alzheimer's disease (AD) and depression. Exposure to elevated temperature (hyperthermia) is proposed as a non-pharmacological, anti-inflammatory treatment for these disorders; however, the underlying mechanisms are not fully understood. Here we asked if the inflammasome, a protein complex essential for orchestrating the inflammatory response and linked to mitochondrial stress, might be modulated by elevated temperatures. To test this, in preliminary studies, immortalized bone-marrow-derived murine macrophages (iBMM) were primed with inflammatory stimuli, exposed to a range of temperatures (37-41.5 °C), and examined for markers of inflammasome and mitochondrial activity. We found that exposure to mild heat stress (39 °C for 15 min) rapidly inhibited iBMM inflammasome activity. Furthermore, heat exposure led to decreased ASC speck formation and increased numbers of polarized mitochondria. These results suggest that mild hyperthermia inhibits inflammasome activity in the iBMM, limiting potentially harmful inflammation and mitigating mitochondrial stress. Our findings suggest an additional potential mechanism by which hyperthermia may exert its beneficial effects on inflammatory diseases.
Keywords: IL-1β; caspase 1; cytokine; hyperthermia; inflammasome; mild heat stress; mitochondria; pyrexia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
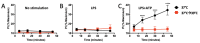

Similar articles
-
Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):127-36. doi: 10.1161/ATVBAHA.114.303763. Epub 2014 Nov 6. Arterioscler Thromb Vasc Biol. 2015. PMID: 25378412
-
HSP70 is a negative regulator of NLRP3 inflammasome activation.Cell Death Dis. 2019 Mar 15;10(4):256. doi: 10.1038/s41419-019-1491-7. Cell Death Dis. 2019. PMID: 30874540 Free PMC article.
-
Inhibition of NLRP3 Inflammasome Activation and Pyroptosis in Macrophages by Taraxasterol Is Associated With Its Regulation on mTOR Signaling.Front Immunol. 2021 Feb 17;12:632606. doi: 10.3389/fimmu.2021.632606. eCollection 2021. Front Immunol. 2021. PMID: 33679781 Free PMC article.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages.J Cell Physiol. 2020 Oct;235(10):7554-7566. doi: 10.1002/jcp.29659. Epub 2020 Mar 1. J Cell Physiol. 2020. PMID: 32115713
Cited by
-
Ti3C2Tx MXene-Decorated 3D-Printed Ceramic Scaffolds for Enhancing Osteogenesis by Spatiotemporally Orchestrating Inflammatory and Bone Repair Responses.Adv Sci (Weinh). 2024 Sep;11(34):e2400229. doi: 10.1002/advs.202400229. Epub 2024 Jul 8. Adv Sci (Weinh). 2024. PMID: 38973266 Free PMC article.
-
Harnessing Hyperthermia: Molecular, Cellular, and Immunological Insights for Enhanced Anticancer Therapies.Integr Cancer Ther. 2024 Jan-Dec;23:15347354241242094. doi: 10.1177/15347354241242094. Integr Cancer Ther. 2024. PMID: 38818970 Free PMC article. Review.
References
-
- Al-Hakeim H.K., Al-Rubaye H.T., Al-Hadrawi D.S., Almulla A.F., Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry. 2023;28:564–578. doi: 10.1038/s41380-022-01836-9. - DOI - PMC - PubMed
-
- Janssen C.W., Lowry C.A., Mehl M.R., Allen J.J., Kelly K.L., Gartner D.E., Medrano A., Begay T.K., Rentscher K., White J.J., et al. Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2016;73:789–795. doi: 10.1001/jamapsychiatry.2016.1031. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

