Perivascular Adipose Tissue and Vascular Smooth Muscle Tone: Friends or Foes?
- PMID: 37190105
- PMCID: PMC10136477
- DOI: 10.3390/cells12081196
Perivascular Adipose Tissue and Vascular Smooth Muscle Tone: Friends or Foes?
Abstract
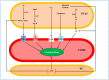
Perivascular adipose tissue (PVAT) is a specialized type of adipose tissue that surrounds most mammalian blood vessels. PVAT is a metabolically active, endocrine organ capable of regulating blood vessel tone, endothelium function, vascular smooth muscle cell growth and proliferation, and contributing critically to cardiovascular disease onset and progression. In the context of vascular tone regulation, under physiological conditions, PVAT exerts a potent anticontractile effect by releasing a plethora of vasoactive substances, including NO, H2S, H2O2, prostacyclin, palmitic acid methyl ester, angiotensin 1-7, adiponectin, leptin, and omentin. However, under certain pathophysiological conditions, PVAT exerts pro-contractile effects by decreasing the production of anticontractile and increasing that of pro-contractile factors, including superoxide anion, angiotensin II, catecholamines, prostaglandins, chemerin, resistin, and visfatin. The present review discusses the regulatory effect of PVAT on vascular tone and the factors involved. In this scenario, dissecting the precise role of PVAT is a prerequisite to the development of PVAT-targeted therapies.
Keywords: PVAT; anti-contractile; endothelial dysfunction; pro-contractile; vascular tone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

