A Recent Review on Cancer Nanomedicine
- PMID: 37190185
- PMCID: PMC10136552
- DOI: 10.3390/cancers15082256
A Recent Review on Cancer Nanomedicine
Abstract
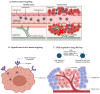
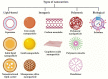
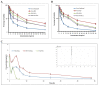
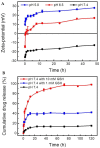
Cancer is one of the most prevalent diseases globally and is the second major cause of death in the United States. Despite the continuous efforts to understand tumor mechanisms and various approaches taken for treatment over decades, no significant improvements have been observed in cancer therapy. Lack of tumor specificity, dose-related toxicity, low bioavailability, and lack of stability of chemotherapeutics are major hindrances to cancer treatment. Nanomedicine has drawn the attention of many researchers due to its potential for tumor-specific delivery while minimizing unwanted side effects. The application of these nanoparticles is not limited to just therapeutic uses; some of them have shown to have extremely promising diagnostic potential. In this review, we describe and compare various types of nanoparticles and their role in advancing cancer treatment. We further highlight various nanoformulations currently approved for cancer therapy as well as under different phases of clinical trials. Finally, we discuss the prospect of nanomedicine in cancer management.
Keywords: cancer; chemotherapy; inorganic nanoparticles; liposomes; nanoparticles; targeted drug delivery; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

