Evaluation of Response to Atezolizumab Plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma Using the Combination of Response Evaluation Criteria in Solid Tumors and Alpha-Fetoprotein
- PMID: 37190231
- PMCID: PMC10137288
- DOI: 10.3390/cancers15082304
Evaluation of Response to Atezolizumab Plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma Using the Combination of Response Evaluation Criteria in Solid Tumors and Alpha-Fetoprotein
Abstract
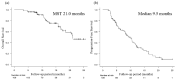
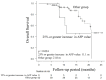
Atezolizumab plus bevacizumab combination therapy (Atezo + Beva) is currently positioned as the first-line therapy for unresectable hepatocellular carcinoma (u-HCC). It may be difficult to decide whether to continue this treatment if radiological response is assessed as stable disease (SD). Therefore, the relationship between radiological response and prognosis was analyzed. A total of 109 patients with u-HCC and Child-Pugh Score of 5-7 received this treatment. Radiological response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST at the first and second evaluations. Of SD patients (n = 71) at the first RECIST evaluation, partial response, SD, and progressive disease (PD) were seen in 10, 55, and 6 patients, respectively, at the second evaluation. On multivariate analysis, in patients with SD at the first RECIST evaluation, a 25% or greater increase in the alpha-fetoprotein (AFP) value from initiation of treatment (odds ratio, 7.38; p = 0.037) was the independent factor for PD at the second evaluation. In patients with SD (n = 59) at the second RECIST evaluation, decreased AFP from initiation of treatment (hazard ratio, 0.46; p = 0.022) was the independent factor related to progression-free survival on multivariate analysis. AFP trends could help decide the Atezo + Beva treatment strategy.
Keywords: Response Evaluation Criteria in Solid Tumors (RECIST); alpha-fetoprotein (AFP); atezolizumab plus bevacizumab combination therapy (Atezo + Beva); hepatocellular carcinoma (HCC); overall survival (OS); progression-free survival (PFS).
Conflict of interest statement
H.A. has received honoraria from Eisai Co., Ltd. and Chugai Pharmaceutical Co., Ltd. Other authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

