Biallelic variants in ribonuclease inhibitor (RNH1), an inflammasome modulator, are associated with a distinctive subtype of acute, necrotizing encephalopathy
- PMID: 37191094
- PMCID: PMC10506156
- DOI: 10.1016/j.gim.2023.100897
Biallelic variants in ribonuclease inhibitor (RNH1), an inflammasome modulator, are associated with a distinctive subtype of acute, necrotizing encephalopathy
Abstract
Purpose: Mendelian etiologies for acute encephalopathies in previously healthy children are poorly understood, with the exception of RAN binding protein 2 (RANBP2)-associated acute necrotizing encephalopathy subtype 1 (ANE1). We provide clinical, genetic, and neuroradiological evidence that biallelic variants in ribonuclease inhibitor (RNH1) confer susceptibility to a distinctive ANE subtype.
Methods: This study aimed to evaluate clinical data, neuroradiological studies, genomic sequencing, and protein immunoblotting results in 8 children from 4 families who experienced acute febrile encephalopathy.
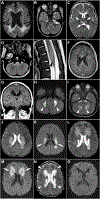
Results: All 8 healthy children became acutely encephalopathic during a viral/febrile illness and received a variety of immune modulation treatments. Long-term outcomes varied from death to severe neurologic deficits to normal outcomes. The neuroradiological findings overlapped with ANE but had distinguishing features. All affected children had biallelic predicted damaging variants in RNH1: a subset that was studied had undetectable RNH1 protein. Incomplete penetrance of the RNH1 variants was evident in 1 family.
Conclusion: Biallelic variants in RNH1 confer susceptibility to a subtype of ANE (ANE2) in previously healthy children. Intensive immunological treatments may alter outcomes. Genomic sequencing in children with unexplained acute febrile encephalopathy can detect underlying genetic etiologies, such as RNH1, and improve outcomes in the probands and at-risk siblings.
Keywords: Acute demyelinating encephalopathy; Acute necrotizing encephalopathy; Inflammasome; RANBP2; RNH1.
Copyright © 2023 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Rebecca Ganetzky is a paid consultant for Minovia Therapeutics and Nurture Genomics. Neal Sondheimer is employed by Synlogic, Inc. All other authors declare no conflicts of interest.
Figures



References
-
- Hedberg-Oldfors C, Mitra S, Molinaro A, et al. Ribonuclease inhibitor 1 (RNH1) deficiency cause congenital cataracts and global developmental delay with infection-induced psychomotor regression and anemia. Eur J Hum Genet Published online March 20, 2023. 10.1038/s41431-023-01327-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

