Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies
- PMID: 37191427
- PMCID: PMC10187711
- DOI: 10.14336/AD.2022.0916
Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies
Abstract
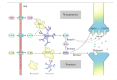
The metabolism of L-tryptophan (TRP) regulates homeostasis, immunity, and neuronal function. Altered TRP metabolism has been implicated in the pathophysiology of various diseases of the central nervous system. TRP is metabolized through two main pathways, the kynurenine pathway and the methoxyindole pathway. First, TRP is metabolized to kynurenine, then kynurenic acid, quinolinic acid, anthranilic acid, 3-hydroxykynurenine, and finally 3-hydroxyanthranilic acid along the kynurenine pathway. Second, TRP is metabolized to serotonin and melatonin along the methoxyindole pathway. In this review, we summarize the biological properties of key metabolites and their pathogenic functions in 12 disorders of the central nervous system: schizophrenia, bipolar disorder, major depressive disorder, spinal cord injury, traumatic brain injury, ischemic stroke, intracerebral hemorrhage, multiple sclerosis, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and Huntington's disease. Furthermore, we summarize preclinical and clinical studies, mainly since 2015, that investigated the metabolic pathway of TRP, focusing on changes in biomarkers of these neurologic disorders, their pathogenic implications, and potential therapeutic strategies targeting this metabolic pathway. This critical, comprehensive, and up-to-date review helps identify promising directions for future preclinical, clinical, and translational research on neuropsychiatric disorders.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
