Macrolides Decrease the Proinflammatory Activity of Macrolide-Resistant Streptococcus pneumoniae
- PMID: 37191519
- PMCID: PMC10269745
- DOI: 10.1128/spectrum.00148-23
Macrolides Decrease the Proinflammatory Activity of Macrolide-Resistant Streptococcus pneumoniae
Abstract
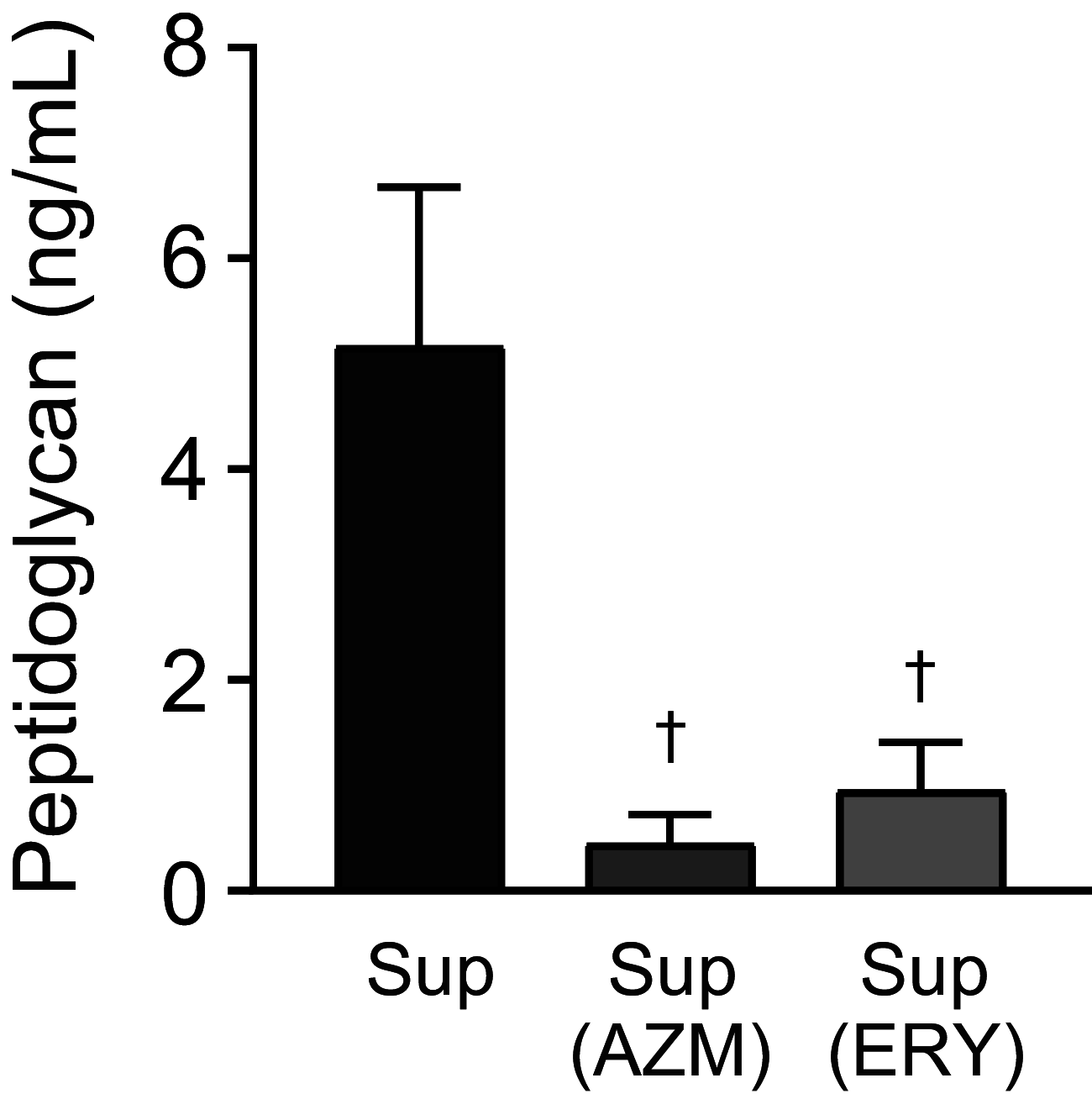
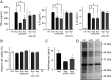
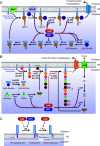
Over the past 2 decades, the prevalence of macrolide-resistant Streptococcus pneumoniae (MRSP) has increased considerably, due to widespread macrolide use. Although macrolide usage has been proposed to be associated with treatment failure in patients with pneumococcal diseases, macrolides may be clinically effective for treating these diseases, regardless of the susceptibility of the causative pneumococci to macrolides. As we previously demonstrated that macrolides downregulate the transcription of various genes in MRSP, including the gene encoding the pore-forming toxin pneumolysin, we hypothesized that macrolides affect the proinflammatory activity of MRSP. Using HEK-Blue cell lines, we found that the supernatants from macrolide-treated MRSP cultures induced decreased NF-κB activation in cells expressing Toll-like receptor 2 and nucleotide-binding oligomerization domain 2 compared to the supernatants from untreated MRSP cells, suggesting that macrolides inhibit the release of these ligands from MRSP. Real-time PCR analysis revealed that macrolides significantly downregulated the transcription of various genes encoding peptidoglycan synthesis-, lipoteichoic acid synthesis-, and lipoprotein synthesis-related molecules in MRSP cells. The silkworm larva plasma assay demonstrated that the peptidoglycan concentrations in the supernatants from macrolide-treated MRSP cultures were significantly lower than those from untreated MRSP cultures. Triton X-114 phase separation revealed that lipoprotein expression decreased in macrolide-treated MRSP cells compared to the lipoprotein expression in untreated MRSP cells. Consequently, macrolides may decrease the expression of bacterial ligands of innate immune receptors, resulting in the decreased proinflammatory activity of MRSP. IMPORTANCE To date, the clinical efficacy of macrolides in pneumococcal disease is assumed to be linked to their ability to inhibit the release of pneumolysin. However, our previous study demonstrated that oral administration of macrolides to mice intratracheally infected with macrolide-resistant Streptococcus pneumoniae resulted in decreased levels of pneumolysin and proinflammatory cytokines in bronchoalveolar lavage fluid samples compared to the levels in samples from untreated infected control mice, without affecting the bacterial load in the fluid. This finding suggests that additional mechanisms by which macrolides negatively regulate proinflammatory cytokine production may be involved in their efficacy in vivo. Furthermore, in this study, we demonstrated that macrolides downregulated the transcription of various proinflammatory-component-related genes in S. pneumoniae, which provides an additional explanation for the clinical benefits of macrolides.
Keywords: inflammation; lipoproteins; lipoteichoic acid; macrolide-resistant Streptococcus pneumoniae; macrolides; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
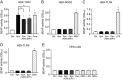
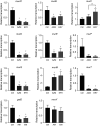
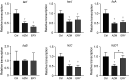
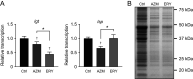

Similar articles
-
Clarithromycin Inhibits Pneumolysin Production via Downregulation of ply Gene Transcription despite Autolysis Activation.Microbiol Spectr. 2021 Oct 31;9(2):e0031821. doi: 10.1128/Spectrum.00318-21. Epub 2021 Sep 1. Microbiol Spectr. 2021. PMID: 34468195 Free PMC article.
-
Mechanism of Macrolide-Induced Inhibition of Pneumolysin Release Involves Impairment of Autolysin Release in Macrolide-Resistant Streptococcus pneumoniae.Antimicrob Agents Chemother. 2018 Oct 24;62(11):e00161-18. doi: 10.1128/AAC.00161-18. Print 2018 Nov. Antimicrob Agents Chemother. 2018. PMID: 30181369 Free PMC article.
-
Macrolide Resistance in Streptococcus pneumoniae.Front Cell Infect Microbiol. 2016 Sep 21;6:98. doi: 10.3389/fcimb.2016.00098. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27709102 Free PMC article. Review.
-
Evolution and molecular characterization of macrolide-resistant Streptococcus pneumoniae in Canada between 1998 and 2008.J Antimicrob Chemother. 2014 Jan;69(1):59-66. doi: 10.1093/jac/dkt332. Epub 2013 Aug 22. J Antimicrob Chemother. 2014. PMID: 23970485
-
The Crisis of Macrolide Resistance in Pneumococci in Latin America.Am J Trop Med Hyg. 2024 Jul 30;111(4):756-764. doi: 10.4269/ajtmh.23-0913. Print 2024 Oct 2. Am J Trop Med Hyg. 2024. PMID: 39084209 Free PMC article. Review.
References
-
- Nagai K, Kimura O, Domon H, Maekawa T, Yonezawa D, Terao Y. 2019. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children with acute otitis media in Japan from 2014 to 2017. J Infect Chemother 25:229–232. doi:10.1016/j.jiac.2018.08.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

