Acinetobacter Metabolism in Infection and Antimicrobial Resistance
- PMID: 37191522
- PMCID: PMC10269061
- DOI: 10.1128/iai.00433-22
Acinetobacter Metabolism in Infection and Antimicrobial Resistance
Abstract
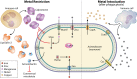
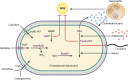
Acinetobacter infections have high rates of mortality due to an increasing incidence of infections by multidrug-resistant (MDR) and extensively-drug-resistant (XDR) strains. Therefore, new therapeutic strategies for the treatment of Acinetobacter infections are urgently needed. Acinetobacter spp. are Gram-negative coccobacilli that are obligate aerobes and can utilize a wide variety of carbon sources. Acinetobacter baumannii is the main cause of Acinetobacter infections, and recent work has identified multiple strategies A. baumannii uses to acquire nutrients and replicate in the face of host nutrient restriction. Some host nutrient sources also serve antimicrobial and immunomodulatory functions. Hence, understanding Acinetobacter metabolism during infection may provide new insights into novel infection control measures. In this review, we focus on the role of metabolism during infection and in resistance to antibiotics and other antimicrobial agents and discuss the possibility that metabolism may be exploited to identify novel targets to treat Acinetobacter infections.
Keywords: A. baumannii; Acinetobacter; antibiotic resistance; antimicrobial; antimicrobial resistance; infection; metabolism; nutrients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cools P, Nemec A, Kämpfer P, Vaneechoutte M. 2019. Acinetobacter, Chryseobacterium, Moraxella, and other nonfermentative Gram-negative rods, p 829–857. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC.
-
- CDC. 2022. COVID-19: U.S. Impact on antimicrobial resistance. Special report 2022. National Center for Emerging and Zoonotic Infectious Diseases, Atlanta, GA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

