Transcriptional Regulation of Autophagy-Related Genes by Sin3 Negatively Modulates Autophagy in Magnaporthe oryzae
- PMID: 37191531
- PMCID: PMC10269650
- DOI: 10.1128/spectrum.00171-23
Transcriptional Regulation of Autophagy-Related Genes by Sin3 Negatively Modulates Autophagy in Magnaporthe oryzae
Abstract
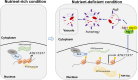
Autophagy is a conserved degradation and recycling pathway in eukaryotes and is important for their normal growth and development. An appropriate status of autophagy is crucial for organisms which is tightly regulated both temporally and continuously. Transcriptional regulation of autophagy-related genes (ATGs) is an important layer in autophagy regulation. However, the transcriptional regulators and their mechanisms are still unclear, especially in fungal pathogens. Here, we identified Sin3, a component of the histone deacetylase complex, as a transcriptional repressor of ATGs and negative regulator of autophagy induction in the rice fungal pathogen Magnaporthe oryzae. A loss of SIN3 resulted in upregulated expression of ATGs and promoted autophagy with an increased number of autophagosomes under normal growth conditions. Furthermore, we found that Sin3 negatively regulated the transcription of ATG1, ATG13, and ATG17 through direct occupancy and changed levels of histone acetylation. Under nutrient-deficient conditions, the transcription of SIN3 was downregulated, and the reduced occupancy of Sin3 from those ATGs resulted in histone hyperacetylation and activated their transcription and in turn promoted autophagy. Thus, our study uncovers a new mechanism of Sin3 in modulating autophagy through transcriptional regulation. IMPORTANCE Autophagy is an evolutionarily conserved metabolic process and is required for the growth and pathogenicity of phytopathogenic fungi. The transcriptional regulators and precise mechanisms of regulating autophagy, as well as whether the induction or repression of ATGs is associated with autophagy level, are still poorly understood in M. oryzae. In this study, we revealed that Sin3 acts as a transcriptional repressor of ATGs to negatively regulate autophagy level in M. oryzae. Under the nutrient-rich conditions, Sin3 inhibits autophagy with a basal level through directly repressing the transcription of ATG1-ATG13-ATG17. Upon nutrient-deficient treatment, the transcriptional level of SIN3 would decrease and dissociation of Sin3 from those ATGs associates with histone hyperacetylation and activates their transcriptional expression and in turn contributes to autophagy induction. Our findings are important as we uncover a new mechanism of Sin3 for the first time to negatively modulate autophagy at the transcriptional level in M. oryzae.
Keywords: ATG8; fungal pathogen; histone deacetylation; rice blast; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae.Autophagy. 2018;14(9):1543-1561. doi: 10.1080/15548627.2018.1458171. Epub 2018 Aug 31. Autophagy. 2018. PMID: 29929416 Free PMC article.
-
The additional PRC2 subunit and Sin3 histone deacetylase complex are required for the normal distribution of H3K27me3 occupancy and transcriptional silencing in Magnaporthe oryzae.New Phytol. 2022 Oct;236(2):576-589. doi: 10.1111/nph.18383. Epub 2022 Aug 3. New Phytol. 2022. PMID: 35842786
-
A Subunit of the COP9 Signalosome, MoCsn6, Is Involved in Fungal Development, Pathogenicity, and Autophagy in Rice Blast Fungus.Microbiol Spectr. 2022 Dec 21;10(6):e0202022. doi: 10.1128/spectrum.02020-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445131 Free PMC article.
-
Co-repressor, co-activator and general transcription factor: the many faces of the Sin3 histone deacetylase (HDAC) complex.Biochem J. 2018 Dec 14;475(24):3921-3932. doi: 10.1042/BCJ20170314. Biochem J. 2018. PMID: 30552170 Free PMC article. Review.
-
Investigation of the biological roles of autophagy in appressorium morphogenesis in Magnaporthe oryzae.J Zhejiang Univ Sci B. 2008 Oct;9(10):793-6. doi: 10.1631/jzus.B0860013. J Zhejiang Univ Sci B. 2008. PMID: 18837106 Free PMC article. Review.
Cited by
-
Two H3K36 methyltransferases differentially associate with transcriptional activity and enrichment of facultative heterochromatin in rice blast fungus.aBIOTECH. 2023 Dec 18;5(1):1-16. doi: 10.1007/s42994-023-00127-3. eCollection 2024 Mar. aBIOTECH. 2023. PMID: 38576437 Free PMC article.
-
Two Subunits of the Rpd3 Histone Deacetylase Complex of Cochliobolus heterostrophus Are Essential for Nitrosative Stress Response and Virulence, and Interact With Stress-Response Regulators ChHog1 and ChCrz1.Mol Plant Pathol. 2025 Aug;26(8):e70131. doi: 10.1111/mpp.70131. Mol Plant Pathol. 2025. PMID: 40773473 Free PMC article.
References
-
- He M, Xu Y, Chen J, Luo Y, Lv Y, Su J, Kershaw MJ, Li W, Wang J, Yin J, Zhu X, Liu X, Chern M, Ma B, Wang J, Qin P, Chen W, Wang Y, Wang W, Ren Z, Wu X, Li P, Li S, Peng Y, Lin F, Talbot NJ, Chen X. 2018. MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae. Autophagy 14:1543–1561. doi:10.1080/15548627.2018.1458171. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

