Remodeled connexin 43 hemichannels alter cardiac excitability and promote arrhythmias
- PMID: 37191672
- PMCID: PMC10192603
- DOI: 10.1085/jgp.202213150
Remodeled connexin 43 hemichannels alter cardiac excitability and promote arrhythmias
Abstract
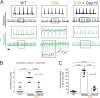
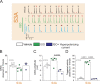
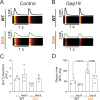
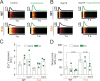
Connexin-43 (Cx43) is the most abundant protein forming gap junction channels (GJCs) in cardiac ventricles. In multiple cardiac pathologies, including hypertrophy and heart failure, Cx43 is found remodeled at the lateral side of the intercalated discs of ventricular cardiomyocytes. Remodeling of Cx43 has been long linked to spontaneous ventricular arrhythmia, yet the mechanisms by which arrhythmias develop are still debated. Using a model of dystrophic cardiomyopathy, we previously showed that remodeled Cx43 function as aberrant hemichannels (non-forming GJCs) that alter cardiomyocyte excitability and, consequently, promote arrhythmias. Here, we aim to evaluate if opening of remodeled Cx43 can serve as a general mechanism to alter cardiac excitability independent of cellular dysfunction associated with a particular cardiomyopathy. To address this issue, we used a genetically modified Cx43 knock-in mouse (S3A) that promotes cardiac remodeling of Cx43 protein without apparent cardiac dysfunction. Importantly, when S3A mice were subjected to cardiac stress using the β-adrenergic agonist isoproterenol (Iso), they displayed acute and severe arrhythmias, which were not observed in WT mice. Pretreatment of S3A mice with the Cx43 hemichannel blocker, Gap19, prevented Iso-induced abnormal electrocardiographic behavior. At the cellular level, when compared with WT, Iso-treated S3A cardiomyocytes showed increased membrane permeability, greater plasma membrane depolarization, and Ca2+ overload, which likely caused prolonged action potentials, delayed after depolarizations, and triggered activity. All these cellular dysfunctions were also prevented by Cx43 hemichannel blockers. Our results support the notion that opening of remodeled Cx43 hemichannels, regardless of the type of cardiomyopathy, is sufficient to mediate cardiac-stress-induced arrhythmogenicity.
© 2023 Lillo et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






References
-
- Abudara, V., Bechberger J., Freitas-Andrade M., De Bock M., Wang N., Bultynck G., Naus C.C., Leybaert L., and Giaume C.. 2014. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front. Cell. Neurosci. 8:306. 10.3389/fncel.2014.00306 - DOI - PMC - PubMed
-
- De Smet, M.A., Lissoni A., Nezlobinsky T., Wang N., Dries E., Pérez-Hernández M., Lin X., Amoni M., Vervliet T., Witschas K., et al. . 2021. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J. Clin. Invest. 131:131. 10.1172/JCI137752 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

