Evaluation of off-label rapamycin use to promote healthspan in 333 adults
- PMID: 37191826
- PMCID: PMC10187519
- DOI: 10.1007/s11357-023-00818-1
Evaluation of off-label rapamycin use to promote healthspan in 333 adults
Abstract
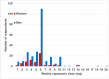
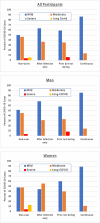
Rapamycin (sirolimus) is an FDA-approved drug with immune-modulating and growth-inhibitory properties. Preclinical studies have shown that rapamycin extends lifespan and healthspan metrics in yeast, invertebrates, and rodents. Several physicians are now prescribing rapamycin off-label as a preventative therapy to maintain healthspan. Thus far, however, there is limited data available on side effects or efficacy associated with use of rapamycin in this context. To begin to address this gap in knowledge, we collected data from 333 adults with a history of off-label use of rapamycin by survey. Similar data were also collected from 172 adults who had never used rapamycin. Here, we describe the general characteristics of a patient cohort using off-label rapamycin and present initial evidence that rapamycin can be used safely in adults of normal health status.
Keywords: Aging; COVID-19; Healthspan; Longevity; Rapamune; SARS-CoV-2; Side effects; Sirolimus.
© 2023. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
MK and GH are co-founders of Optispan Geroscience, which has no material interest in rapamycin or rapamycin derivative production or sales at this time. ASG and BSR are practicing physicians who may prescribe rapamycin off-label for certain patients when appropriate. AI, AN, and SZ are employees and/or shareholders of AgelessRX, a longevity-focused telemedicine platform that sponsors several clinical trials, including interventional and observational efforts for the repurposed use of rapamycin.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

