The regulation and functions of the matricellular CCN proteins induced by shear stress
- PMID: 37191841
- PMCID: PMC10326207
- DOI: 10.1007/s12079-023-00760-z
The regulation and functions of the matricellular CCN proteins induced by shear stress
Abstract
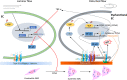
Shear stress is a frictional drag generated by the flow of fluid, such as blood or interstitial fluid, and plays a critical role in regulating cellular gene expression and functional phenotype. The matricellular CCN family proteins are dynamically regulated by shear stress of different flow patterns, and their expression significantly alters the microenvironment of cells. Secreted CCN proteins mainly bind to several cell surface integrin receptors to mediate their diverse functions in regulating cell survival, function, and behavior. Gene-knockout studies indicate major functions of CCN proteins in the cardiovascular and skeletal systems, the two primary systems in which CCN expressions are regulated by shear stress. In the cardiovascular system, the endothelium is directly exposed to vascular shear stress. Unidirectional laminar blood flow generates laminar shear stress, which promotes a mature endothelial phenotype and upregulates anti-inflammatory CCN3 expression. In contrast, disturbed flow generates oscillatory shear stress, which induces endothelial dysfunction through the induction of CCN1 and CCN2. Shear-induced CCN1 binds to integrin α6β1 and promotes superoxide production, NF-κB activation, and inflammatory gene expression in endothelial cells. Although the interaction between shear stress and CCN4-6 is not clear, CCN 4 exhibits a proinflammatory property and CCN5 inhibits vascular cell growth and migration. The crucial roles of CCN proteins in cardiovascular development, homeostasis, and disease are evident but not fully understood. In the skeletal system, mechanical loading on bone generates shear stress from interstitial fluid in the lacuna-canalicular system and promotes osteoblast differentiation and bone formation. CCN1 and CCN2 are induced and potentially mediate fluid shear stress mechanosensing in osteocytes. However, the exact roles of interstitial shear stress-induced CCN1 and CCN2 in bone are still not clear. In contrast to other CCN family proteins, CCN3 inhibits osteoblast differentiation, although its regulation by interstitial shear stress in osteocytes has not been reported. The induction of CCN proteins by shear stress in bone and their functions remain largely unknown and merit further investigation. This review discusses the expression and functions of CCN proteins regulated by shear stress in physiological conditions, diseases, and cell culture models. The roles between CCN family proteins can be compensatory or counteractive in tissue remodeling and homeostasis.
Keywords: Bone remodeling; CCN proteins; Endothelial dysfunction; Lacuna-canalicular system; Shear stress.
© 2023. The International CCN Society.
Figures

References
-
- Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, Chavakis T. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

