Continuous Data-Driven Monitoring in Critical Congenital Heart Disease: Clinical Deterioration Model Development
- PMID: 37191988
- PMCID: PMC10230358
- DOI: 10.2196/45190
Continuous Data-Driven Monitoring in Critical Congenital Heart Disease: Clinical Deterioration Model Development
Abstract
Background: Critical congenital heart disease (cCHD)-requiring cardiac intervention in the first year of life for survival-occurs globally in 2-3 of every 1000 live births. In the critical perioperative period, intensive multimodal monitoring at a pediatric intensive care unit (PICU) is warranted, as their organs-especially the brain-may be severely injured due to hemodynamic and respiratory events. These 24/7 clinical data streams yield large quantities of high-frequency data, which are challenging in terms of interpretation due to the varying and dynamic physiology innate to cCHD. Through advanced data science algorithms, these dynamic data can be condensed into comprehensible information, reducing the cognitive load on the medical team and providing data-driven monitoring support through automated detection of clinical deterioration, which may facilitate timely intervention.
Objective: This study aimed to develop a clinical deterioration detection algorithm for PICU patients with cCHD.
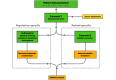
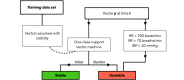
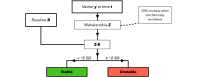
Methods: Retrospectively, synchronous per-second data of cerebral regional oxygen saturation (rSO2) and 4 vital parameters (respiratory rate, heart rate, oxygen saturation, and invasive mean blood pressure) in neonates with cCHD admitted to the University Medical Center Utrecht, the Netherlands, between 2002 and 2018 were extracted. Patients were stratified based on mean oxygen saturation during admission to account for physiological differences between acyanotic and cyanotic cCHD. Each subset was used to train our algorithm in classifying data as either stable, unstable, or sensor dysfunction. The algorithm was designed to detect combinations of parameters abnormal to the stratified subpopulation and significant deviations from the patient's unique baseline, which were further analyzed to distinguish clinical improvement from deterioration. Novel data were used for testing, visualized in detail, and internally validated by pediatric intensivists.
Results: A retrospective query yielded 4600 hours and 209 hours of per-second data in 78 and 10 neonates for, respectively, training and testing purposes. During testing, stable episodes occurred 153 times, of which 134 (88%) were correctly detected. Unstable episodes were correctly noted in 46 of 57 (81%) observed episodes. Twelve expert-confirmed unstable episodes were missed in testing. Time-percentual accuracy was 93% and 77% for, respectively, stable and unstable episodes. A total of 138 sensorial dysfunctions were detected, of which 130 (94%) were correct.
Conclusions: In this proof-of-concept study, a clinical deterioration detection algorithm was developed and retrospectively evaluated to classify clinical stability and instability, achieving reasonable performance considering the heterogeneous population of neonates with cCHD. Combined analysis of baseline (ie, patient-specific) deviations and simultaneous parameter-shifting (ie, population-specific) proofs would be promising with respect to enhancing applicability to heterogeneous critically ill pediatric populations. After prospective validation, the current-and comparable-models may, in the future, be used in the automated detection of clinical deterioration and eventually provide data-driven monitoring support to the medical team, allowing for timely intervention.
Keywords: aberration detection; artificial intelligence; cardiac monitoring; classification model; clinical deterioration; congenital heart disease; machine learning; paediatric intensive care; pediatric intensive care; peri-operative; perioperative; surgery.
©Ruben S Zoodsma, Rian Bosch, Thomas Alderliesten, Casper W Bollen, Teus H Kappen, Erik Koomen, Arno Siebes, Joppe Nijman. Originally published in JMIR Cardio (https://cardio.jmir.org), 16.05.2023.
Conflict of interest statement
Conflicts of Interest: EK has received consulting or speaker honorarium from Philips, GE healthcare, Getinge, and B Braun in the past. The other authors declare that they have no competing interests.
Figures
References
-
- Oster Matthew E, Lee K, Honein M, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013 May;131(5):e1502–e1508. doi: 10.1542/peds.2012-3435. https://europepmc.org/abstract/MED/23610203 peds.2012-3435 - DOI - PMC - PubMed
-
- Nicoll Jessica, Somer J, Eytan D, Chau V, Marini D, Lim J, Greer Robert, Aly Safwat, Seed Mike, Miller Steven P, Laussen Peter C, Mazwi Mjaye L, Schwartz Steven M. Analyzing continuous physiologic data to find hemodynamic signatures associated with new brain injury after congenital heart surgery. Crit Care Explor. 2022 Sep;4(9):e0751. doi: 10.1097/CCE.0000000000000751. https://europepmc.org/abstract/MED/36082376 - DOI - PMC - PubMed
-
- Fister Petja, Robek D, Paro-Panjan D, Mazić Uroš, Lenasi H. Decreased tissue oxygenation in newborns with congenital heart defects: a case-control study. Croat Med J. 2018 Apr 30;59(2):71–78. doi: 10.3325/cmj.2018.59.71. https://europepmc.org/abstract/MED/29740991 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

