Preclinical characterization of ISB 1342, a CD38 × CD3 T-cell engager for relapsed/refractory multiple myeloma
- PMID: 37192303
- PMCID: PMC10644056
- DOI: 10.1182/blood.2022019451
Preclinical characterization of ISB 1342, a CD38 × CD3 T-cell engager for relapsed/refractory multiple myeloma
Abstract
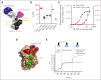
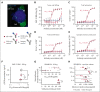
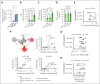
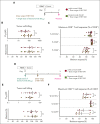
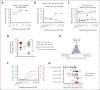
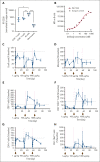
Although treatment of multiple myeloma (MM) with daratumumab significantly extends the patient's lifespan, resistance to therapy is inevitable. ISB 1342 was designed to target MM cells from patients with relapsed/refractory MM (r/r MM) displaying lower sensitivity to daratumumab. ISB 1342 is a bispecific antibody with a high-affinity Fab binding to CD38 on tumor cells on a different epitope than daratumumab and a detuned scFv domain affinity binding to CD3ε on T cells, to mitigate the risk of life-threatening cytokine release syndrome, using the Bispecific Engagement by Antibodies based on the TCR (BEAT) platform. In vitro, ISB 1342 efficiently killed cell lines with different levels of CD38, including those with a lower sensitivity to daratumumab. In a killing assay where multiple modes of action were enabled, ISB 1342 showed higher cytotoxicity toward MM cells compared with daratumumab. This activity was retained when used in sequential or concomitant combinations with daratumumab. The efficacy of ISB 1342 was maintained in daratumumab-treated bone marrow patient samples showing lower sensitivity to daratumumab. ISB 1342 induced complete tumor control in 2 therapeutic mouse models, unlike daratumumab. Finally, in cynomolgus monkeys, ISB 1342 displayed an acceptable toxicology profile. These data suggest that ISB 1342 may be an option in patients with r/r MM refractory to prior anti-CD38 bivalent monoclonal antibody therapies. It is currently being developed in a phase 1 clinical study.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.P., C.E., P.S., E.N., A.L., T.M., D.P.F., A.D., L.C.-I., J.M., M. Pihlgren, M.-A.D., G.S.G., V.M., A.S., E.Z., M. Perro, and M.C. are employees of Ichnos Sciences. M. Pihlgren has shares in AC Immune SA. The remaining authors declare no competing financial interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Usmani S, Ahmadi T, Ng Y, et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;21(11):1355–1361. - PMC - PubMed
-
- Overdijk MB, Jansen JHM, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor–mediated cross-linking. J Immunol. 2016;197(3):807–813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

